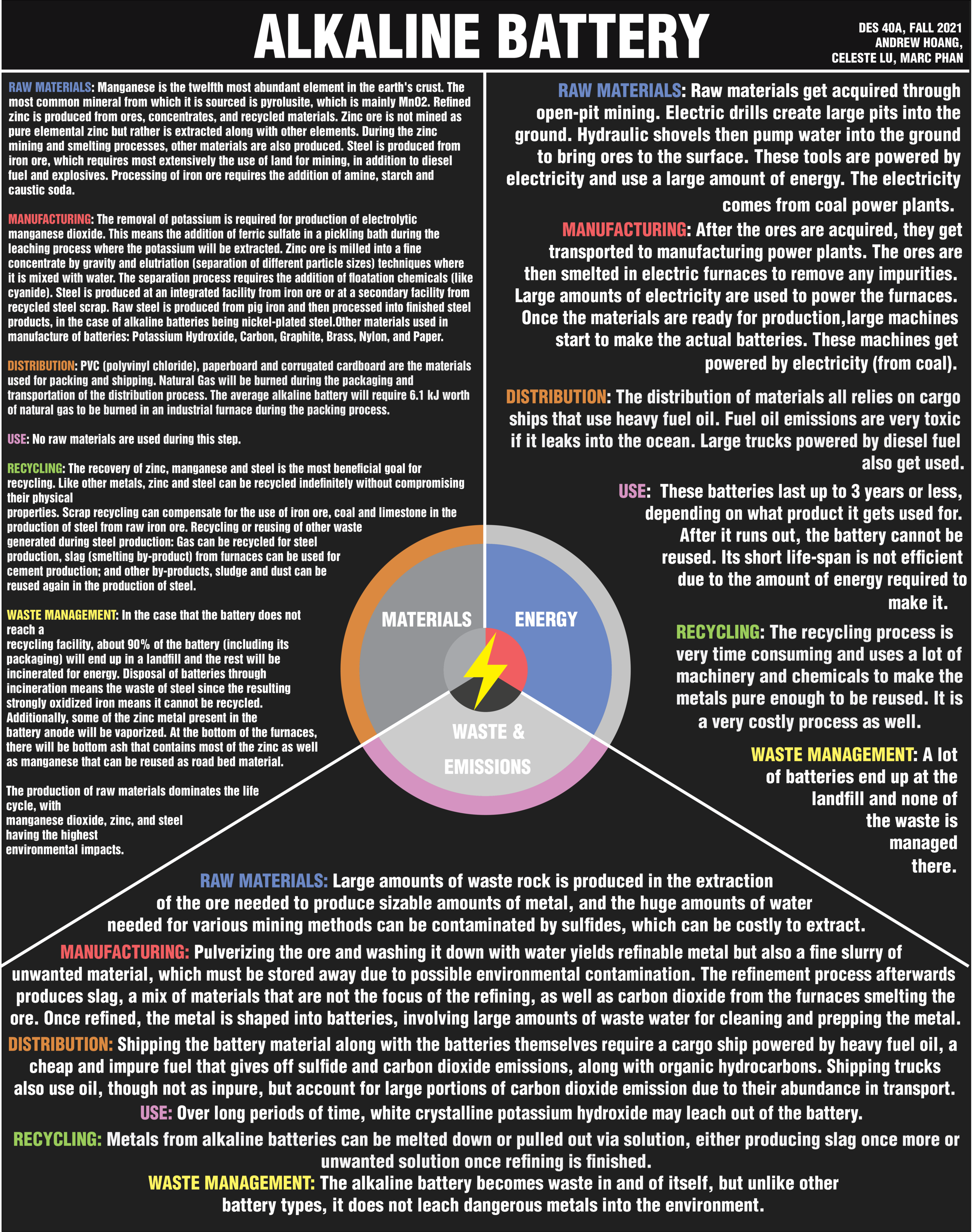
Design Life-Cycle
assess.design.(don't)consume
Andrew Hoang
Professor Cogdell
DES 040A
2 December 2021
Raw Materials of an Alkaline Battery
Alkaline batteries power many items that are part of our everyday life, such as our remotes, flashlights, clocks, etc. Yet not many people know what exactly is contained inside a battery, nor what happens once their battery has run out of energy and been thrown in the bin. A battery provides power by converting the energy from a chemical reaction into electrical energy. Once the chemical reaction can no longer continue, the battery has been “drained” and will not produce power. Then comes the issue of whether disposal or recycling of the battery will be more beneficial. The current life cycle of alkaline batteries is unsustainable and an inefficient use of the raw materials involved.
The production of raw materials dominates the life cycle, with manganese dioxide, zinc, and steel having the highest environmental impacts. These materials happen to be those that make up the greatest part of the composition of an alkaline battery, about seventy-five percent of the average weighted alkaline battery (Olivetti et al.). In regards to the other steps of the life cycle that have comparatively minimal environmental impact, distribution which involves packing and shipping is a simple step relative to production as it does not require as many extensive processes that are required to obtain and prepare raw materials for use in manufacture. No additional raw materials are added during the use of alkaline batteries since they are essentially plug-and-play products. The recycling process can be valuable for offsetting the amount of resources involved in solely the production of raw materials by recovering manganese, zinc, and steel to be reused for other products or industries. However, this heavily depends on the efficiency of the method of recycling, as well as the portion of batteries that actually reach these recycling facilities. Those batteries that do not end up being recycled move on to the step of waste management, where they are thrown into landfills to decay and leak or are incinerated for energy, compromising the physical properties of their components. Therefore, in order to improve the sustainability of alkaline batteries, it is necessary to focus on harvesting their raw materials from recycling, making the production of these materials less cumbersome on the environment, or finding an alternative altogether, whether that be for the raw materials or alkaline batteries themselves.
Zinc has potential as a target for increasing sustainability as one of the big three materials involved in an alkaline battery. Zinc ore is not mined as pure elemental zinc but rather is extracted along with other elements (Van Genderen et al.). During the zinc mining and smelting processes, other materials are also produced. These are lead and copper concentrates that are produced alongside the zinc concentrate as the ore that was mined is processed. When the zinc concentrate is processed into special high grade zinc during smelting, sulfuric acid is produced from the sulfur dioxide that is emitted when the ore is being roasted. The sulfuric acid is either sold or used in other processes carried out within the ore processing facility. So the energy and resources involved in the production of zinc is slightly offset by the production of other co-products. Yet, the consumption of electricity during ore processing and the burning of diesel during mining hold enough significance to lessen the value of these co-products. Because zinc is a metal, it can be recycled indefinitely. Recycled zinc that cannot be used as scrap due to its particle size has been found useful in other industries depending on its properties (Ebin et al.). Metallic zinc particles are used as a pigment for anticorrosive coatings and zinc-air battery electrodes. Another material that can be recycled indefinitely has a place as one of the main materials in a battery.
Steel, which makes up a large portion of the composition of a battery, has many other uses that warrant recycling. Steel can be recycled indefinitely, in fact recycled steel scrap is commonly used for the production of steel by secondary facilities (Yellishetty et al.). Scrap recycling can replace the use of over 1200 kg of iron ore, 7 kg of coal and 51 kg of limestone for a tonne of steel scrap used according to an estimate by the World Steel Organization. So it is clear that as one of the main materials used in alkaline batteries that recycling of steel should be a target, not only due to the potential savings in terms of resources, but also energy that would be saved. This is because the process for manufacturing steel from recycled scrap eliminates the most energy intensive step of raw steel production from iron, the reduction of iron ore in a blast furnace. However, when spent batteries are not received by recycling facilities but instead sent to landfills for incineration, all of the resources and energy that were consumed by the production of their steel shell is wasted. When the batteries are incinerated, the steel can be found in the bottom ash, but the iron will be strongly oxidized (Olivetti et al.). This results in the recycling process for the scrap to be much more energy intensive, lowering the efficiency for recovering the same amount of product. In the demand for steel, manganese metal has also experienced a boom in demand as it is one of the alloying elements used in steelmaking.
Manganese is the main material that makes up a battery, yet since it is also in high demand by other industries it will eventually become scarce. This demand worldwide for manganese has been heavily spurred on by increasing steel production, especially in China (Zhang and Cheng). Manganese is the twelfth most abundant element in the earth's crust, which may seem like an advantage considering its usefulness across various industries, but it also promotes unsustainable usage if manufacturers all share the same short-term mindset of using manganese in their products without contributing resources toward recycling it. Currently, manganese is recycled for the purpose of separation in hydrometallurgical and pyrometallurgical processes (Ebin et al.). These are processes that extract and purify metal from ore. However, recycled manganese oxide powder is also useful for application as a paint pigment, a ceramic colorant, a catalyst for the chemicals industry, as well as a fertilizer. In the case that a battery is incinerated, the bottom ash will contain manganese that can be reused as road bed material (Olivetti et al.). With the high demand for the main materials that make up alkaline batteries, it seems obvious that recycling should be utilized.
It can be more costly, both economically and environmentally, to recycle batteries and reuse their materials than to just dispose of them. An assessment of the life cycle of a battery by the environmental consultant ERM determined that there are positive effects for the environment when batteries are recycled. Unfortunately, the negative effects that come with the collection and transportation of batteries for recycling outweigh these benefits. (Bernardes et al.). New methods of collection where spent batteries are collected alongside other waste materials and are separated afterward for recycling can help lower the environmental impacts of collection. Assuming collection does not negate the advantages, the recycling process itself is oftentimes very energy intensive, inefficient, and even a source for more waste materials and harmful emissions. Recycling facilities that recover metals for reuse will still lose a portion of the metals as slag, with treatment requiring many purification processes that negate the benefits. The paper and plastics from the packaging will also be burned for electricity, releasing toxic chemicals and greenhouse gases (Turner and Nugent). As for the pyrometallurgical process of recovering what metals these facilities do end up obtaining for reuse, they are very energy intensive in addition to requiring systems to collect dust and gas emissions (Almquist et al.). With the current processes that make up the life cycle of an alkaline battery, it is clear that whatever the end of the cycle looks like there will be environmental damage.
The rate of use and recycling for the raw materials involved in an alkaline battery’s life cycle will eventually lead to exhaustion of sources for these materials in addition to high waste emissions. This seems to be the nature of products that involve materials that undergo chemical changes during their use. The appearance of the batteries themselves also lend to further weakening of awareness of how we get our energy as the reactions involved in producing electricity are concealed behind the battery casing.
Works Cited
Almeida, Manuel F., et al. “Characterization of Spent AA Household Alkaline Batteries.” Waste Management, vol. 26, no. 5, Jan. 2006, pp. 466–476, 10.1016/j.wasman.2005.04.005. Accessed 10 Nov. 2021.
Almquist, Catherine, et al. “Metal Sorption Using Manganese Oxides from Alkaline Battery Manufacturing and Postconsumer Wastes.” Journal of Environmental Engineering, vol. 144, no. 9, Sept. 2018, p. 04018090, 10.1061/(asce)ee.1943-7870.0001433. Accessed 21 Oct. 2021.
Bernardes, A.M, et al. “Recycling of Batteries: A Review of Current Processes and Technologies.” Journal of Power Sources, vol. 130, no. 1-2, May 2004, pp. 291–298, www.sciencedirect.com/science/article/abs/pii/S0378775303012230, 10.1016/j.jpowsour.2003.12.026. Accessed 9 Nov. 2021.
Cucchiella, Federica, et al. “Implementation of a Real Option in a Sustainable Supply Chain: An Empirical Study of Alkaline Battery Recycling.” International Journal of Systems Science, vol. 45, no. 6, 21 Jan. 2013, pp. 1268–1282, 10.1080/00207721.2012.761458. Accessed 21 Oct. 2021.
Ebin, Burçak, et al. “Production of Zinc and Manganese Oxide Particles by Pyrolysis of Alkaline and Zn–c Battery Waste.” Waste Management, vol. 51, 1 May 2016, pp. 157–167, www.sciencedirect.com/science/article/pii/S0956053X1530177X, 10.1016/j.wasman.2015.10.029. Accessed 21 Oct. 2021.
Energizer. “What Is in a Battery | Energizer.” Energizer.com, 2019, www.energizer.com/about-batteries/what-is-in-a-battery. Accessed 15 Oct. 2021.
Ferreira, Hélio, and Mariangela Garcia Praça Leite. “A Life Cycle Assessment Study of Iron Ore Mining.” Journal of Cleaner Production, vol. 108, Dec. 2015, pp. 1081–1091, 10.1016/j.jclepro.2015.05.140. Accessed 1 Dec. 2021.
Kanemaru, Takashi, and Toshio Matsuoka. “General Overview of Battery Waste Management in Japan.” Journal of Power Sources, vol. 57, no. 1, 1 Sept. 1995, pp. 23–26, www.sciencedirect.com/science/article/pii/0378775395022333, 10.1016/0378-7753(95)02233-3. Accessed 21 Oct. 2021.
Nagaraj, D. R., and R. S. Farinato. “Evolution of Flotation Chemistry and Chemicals: A Century of Innovations and the Lingering Challenges.” Minerals Engineering, vol. 96-97, 1 Oct. 2016, pp. 2–14, www.sciencedirect.com/science/article/pii/S0892687516301704, 10.1016/j.mineng.2016.06.019. Accessed 1 Dec. 2021.
Nidheesh, P.V., and M. Suresh Kumar. “An Overview of Environmental Sustainability in Cement and Steel Production.” Journal of Cleaner Production, vol. 231, Sept. 2019, pp. 856–871, www.sciencedirect.com/science/article/pii/S0959652619317871, 10.1016/j.jclepro.2019.05.251. Accessed 30 Nov. 2021.
Olivetti, Elsa, et al. LIFE CYCLE IMPACTS of ALKALINE BATTERIES with a FOCUS on END-OF-LIFE a STUDY CONDUCTED for the NATIONAL ELECTRICAL MANUFACTURERS ASSOCIATION. Massachusetts Institute of Technology, Feb. 2011.
Rah, Elisa. Excellent Supply Chains in the Consumer Packaged Goods Industry. 2005.
Sayilgan, E., et al. “A Review of Technologies for the Recovery of Metals from Spent Alkaline and Zinc–Carbon Batteries.” Hydrometallurgy, vol. 97, no. 3, 1 July 2009, pp. 158–166, www.sciencedirect.com/science/article/pii/S0304386X0900053X, 10.1016/j.hydromet.2009.02.008. Accessed 21 Oct. 2021.
Spanos, Constantine, et al. “Life-Cycle Analysis of Flow-Assisted Nickel Zinc-, Manganese Dioxide-, and Valve-Regulated Lead-Acid Batteries Designed for Demand-Charge Reduction.” Renewable and Sustainable Energy Reviews, vol. 43, Mar. 2015, pp. 478–494, www.sciencedirect.com/science/article/abs/pii/S1364032114008971?via%3Dihub, 10.1016/j.rser.2014.10.072. Accessed 15 Oct. 2021.
Turner, James Morton, and Leah M. Nugent. “Charging up Battery Recycling Policies: Extended Producer Responsibility for Single-Use Batteries in the European Union, Canada, and the United States.” Journal of Industrial Ecology, vol. 20, no. 5, 2 Nov. 2015, pp. 1148–1158, 10.1111/jiec.12351. Accessed 22 Oct. 2021.
Van Genderen, Eric, et al. “A Global Life Cycle Assessment for Primary Zinc Production.” The International Journal of Life Cycle Assessment, vol. 21, no. 11, 31 May 2016, pp. 1580–1593, 10.1007/s11367-016-1131-8. Accessed 30 Nov. 2021.
Yellishetty, Mohan, et al. “Environmental Life-Cycle Comparisons of Steel Production and Recycling: Sustainability Issues, Problems and Prospects.” Environmental Science & Policy, vol. 14, no. 6, Oct. 2011, pp. 650–663, 10.1016/j.envsci.2011.04.008. Accessed 30 Nov. 2021.
Yeşiltepe, Selçuk, et al. “Recycling of Alkaline Batteries via a Carbothermal Reduction Process.” Batteries, vol. 5, no. 1, 19 Mar. 2019, p. 35, 10.3390/batteries5010035. Accessed 21 Oct. 2021.
Zhang, Wensheng, and Chu Yong Cheng. “Manganese Metallurgy Review. Part I: Leaching of Ores/Secondary Materials and Recovery of Electrolytic/Chemical Manganese Dioxide.” Hydrometallurgy, vol. 89, no. 3-4, Dec. 2007, pp. 137–159, www.sciencedirect.com/science/article/pii/S0304386X0700179X, 10.1016/j.hydromet.2007.08.010. Accessed 22 Oct. 2021.
Celeste Lu
Professor Cogdell
DES 040A
2 December 2021
Energy Life Cycle of a Disposable Alkaline Battery
There are over 86,000 tons of disposable alkaline batteries that are purchased yearly in the United States. With a market size of 7.48 billion USD, this product is in high demand (Fortune Business Insights). These batteries provide a promising option for powering electronics, especially at their low prices. Since it excels at affordability and efficiency, the demand for these batteries is quite substantial. The limiting factor is that the energy inputs and their harmful environmental effects are costly and outweigh the benefits of the product’s versatility and accessibility. The large amounts of energy consumption necessary to obtain, manufacture, transport, and recycle the materials are highly dependent on fossil fuels. So, while battery companies say their goals are focused on sustainability, it is crucial to analyze each part of the product’s life cycle to see if it is truly environmentally friendly.
The life cycle of the alkaline disposable battery starts with the extraction of raw materials needed to make the product. Most of the energy-intensive processes take place during mining and extracting primary materials. The main materials required to create the batteries are manganese dioxide, potassium hydroxide, zinc, and nickel-plated steel. The first step is that the ores have to be mined and extracted. Open-pit mining is the process where most of the materials (manganese ore, zinc, lead, nickel, and brass) get acquired. This process is when electric-powered drills create large pits into the ground. These pits are hundreds of feet deep to reach the ores necessary for production. The ore gets brought to the surface with hydraulic shovels that pump water into the ground. Both of these processes require large amounts of energy. While electricity is energy that the machines use, it is not clean fuel. It is not a clean fuel because the electricity used comes from coal-powered plants. Since large amounts of electricity are used during the mining and extracting process, it has a large carbon footprint. Over 94% of the life cycle’s energy consumption comes from the mining and extraction phase (Hamade).
Once these materials are acquired, they get transferred to the manufacturing plants. Trucks powered by diesel fuel transport materials to different parts of the country. However, not all primary materials are from one country, so many get imported from others. Large cargo ships powered by petroleum products are necessary to transport materials to manufacturing countries. Mining, extracting, and transporting raw materials are energy-intensive and heavily reliant on fossil fuels.
The next step is refining and processing the materials. Most of the process is said to use fewer fossil fuels due to the usage of electricity. However, while electricity is a clean fuel source, it is created by fossil fuels. China is the location in which most primary materials get processed. The manufacturing and processing begin with smelting the ore. The ore has to undergo smelting at high temperatures for the removal of impurities. These ores go into electric furnaces where extreme amounts of heat melt off any impurities. Large amounts of electricity are necessary during the refining process. The use of electric furnaces is said to be more environmentally friendly compared to coal-fueled blast furnaces. However, most of the electricity produced in China is through coal power plants- which have an extremely high carbon footprint. Therefore, these large factories filled with electric blast furnaces need fossil fuels. The overall refining process is heavily dependent on fossil fuels.
After the materials get refined, they get transported to factories where the manufacturing process takes place. These materials get sent to factories in the United States and other countries for production. These other factories can start production now that the materials are in a usable form. Large cargo ships that use heavy fuel oil are how the materials get transported. Fuel oil is a very thick petroleum product that can power ships that travel around the world. This oil is very toxic when exposed to water and sunlight. Due to its high sulfur contents, if it leaks into the ocean, it can harm aquatic life. It is also a flammable product since it is a petroleum byproduct (Degnarain). Loads of fuel are obligatory for the frequent transportation of materials worldwide. Thus, even before the battery is made, there are already numerous amounts of energy-intensive processes.
When materials reach the manufacturing factories, the production can finally begin. Factories found in the United States are in Illinois, Connecticut, Wisconsin, and Alabama (GlobalSpec). The process of making the battery is not as time-consuming compared to the past stages. Since the materials have already been processed and refined, it is easy to assemble the battery. However, there are many steps and energy usage that go into battery production. Large machines cut the nickel-plated steel and form it into the battery container. The remaining materials get inserted into the containers. The whole manufacturing process is run by machines, running on electricity. Most of the states where batteries get manufactured use coal as their electricity fuel source. Except for Illinois, where nuclear energy is the primary energy source (US Energy Information Administration). This process is also the second most energy-intensive process right after the mining and extraction phase. Since the machines require a sufficient amount of energy to run, most of the process needs fossil fuels. While there are factories in Illinois that may use nuclear energy, the other factories are still dependent on coal. Even if the manufacturing process starts shifting to renewable sources, the previous stages are still heavily reliant on fossil fuels. Therefore, the whole production process is not sustainable due to its heavy usage of nonrenewable fuel sources.
The next part of the life cycle is the transportation of the batteries to the storefronts and households. When the batteries are ready to be sold, they are packaged and transported inside large cargo trucks. These trucks bring manufactured goods to stores all over the country. Some batteries get shipped to other parts of the world with cargo ships and even airplanes. Trucks powered by diesel fuel are the primary mode of transportation. Diesel fuel emits a lot of dangerous emissions after being burned. While it has less pollution compared to regular gasoline, it is not a clean source of energy. Once the batteries are bought and used, the shelf life of the batteries can last from a few months to several years. All of this depends on the purpose of the batteries. If the batteries are powering a clock, then it can last up to 3 years. However, if the batteries are powering a toy or electronic, it can last a few months. After analyzing the various stages, the usability of the battery is not efficient due to the amount of energy and time it takes to make the product.
The life cycle of the battery does not end after the battery’s shelf life runs out. It continues when the battery is either disposed of or recycled. All states except for California permits the consumer to dispose of the batteries into landfills. Many of the alkaline batteries are not being recycled and disposed of properly, because of lenient regulations. Since alkaline batteries made today do not include lead, many states label it as safe for disposal. However, these batteries are unsafe due to other toxic substances that enter the environment due to runoff. If the runoff gets into water streams, the toxins can harm animals and humans. Since there are such lenient regulations, people would rather throw their batteries away instead of bringing them to the battery recycling centers. A substantial amount of used batteries goes to the landfill. So, small amounts of energy get used during the disposal of batteries since they get put into landfills.
For the batteries that make it to the recycling plant, there are several steps to recycle material. The first step is to separate all the materials by milling. Milling is a physical process run by machines to take apart the battery. The next step is acid and base leaching which is when the metals go into a solution. These metals need cleaning before reutilizing for future products. The substances once again undergo further separation in an open furnace. High heat gets used to remove any water and plastic remains. The disadvantage of recycling this battery is how pure the materials need to be. Once it is recycled, it needs to undergo many stages for the materials to be reusable. Overall, the recycling process uses a substantial amount of energy to extract reusable materials from the battery. It is also not economically feasible due to the amount of time and high energy consumption necessary. While it is good that certain metals and substances get reused in future batteries, there is a lot of fuel consumption required for the process to occur.
After a thorough analysis of the alkaline battery’s life cycle, the conclusion is that this product is not energy efficient and sustainable for the environment. While the product is very affordable and accessible for consumers, the product’s energy consumption is too expensive for the environment. The whole embodied life cycle is heavily dependent on fossil fuels and nonrenewable fuel sources. These fuel sources are contributors to climate change due to their high greenhouse gas emissions. Given only a three-year battery lifetime, this battery is too expensive environmentally wise. The regulations placed around battery disposal are far too lenient. So, batteries that make it to the landfill can hurt animals and even humans. Therefore, it is better to find other alternatives to power electronics and household appliances without a high carbon footprint.
Bibliography
“Alkaline Battery Market Size, Share and Covid-19 Impact Analysis, by Product (Primary and Secondary), by Size (AA, AAA, 9 Volt, and Others), by Application (Remote Control, Consumer Electronics, Toys & Radios, and Others), and Regional Forecast, 2021-2028.” Alkaline Battery Market Size, Share | Global Industry Growth, 2028, https://www.fortunebusinessinsights.com/alkaline-battery-market-103298. Accessed 12 Nov. 2021.
“Battery Assembly Machines Suppliers in United States.”, https://www.globalspec.com/local/5691/C_US. Accessed 29 Nov. 2021.
“China’s Power & Steel Firms Continue to Invest in Coal Even as Emissions Surge Cools down.” Energyandcleanair.Org , https://energyandcleanair.org/wp/wp-content/uploads/2021/08/China-Q2-briefing-coal-steel-CO2.pdf . Accessed 12 Nov. 2021.
Comstock, Owen. “Coal Is the Most-Used Electricity Generation Source in 18 States; Natural Gas in 16 - Today in Energy - U.S. Energy Information Administration (EIA).” Homepage - U.S. Energy Information Administration (EIA), https://www.eia.gov/todayinenergy/detail.php?id=37034. Accessed 12 Nov. 2021.
“Digging Deeper: Mining Methods Explained | Anglo American.” Re-Imagining Mining to Improve People’s Lives | Anglo American, https://www.angloamerican.com/futuresmart/stories/our-industry/mining-explained/digging-deeper-mining-methods-explained. Accessed 12 Nov. 2021.
Downing, James. “Manganese Processing.” Encyclopædia Britannica, Encyclopædia Britannica, https://www.britannica.com/technology/manganese-processing. Accessed 12 Nov. 2021.
Espinosa, Denise, and Andrea Bernardes. “An Overview on the Current Processes for the Recycling of Batteries - ScienceDirect.” Science Direct , https://reader.elsevier.com/reader/sd/pii/S0378775304005397?token=121DB4A7A84691D17F4A49BF3CDFCF4766CAD38EC175F4F6E2115DDB1D9289FD0B075F0857858E2FFE8FAE0DBC023155&originRegion=us-east-1&originCreation=20211103235107. Accessed 12 Nov. 2021.
Fritt-Rasmussen, Janne, et al. Heavy Fuel Oil . https://norden.diva-portal.org/smash/get/diva2:1259220/FULLTEXT01.pdf . Accessed 12 Nov. 2021.
Hamade, Ramsey, et al. “Life Cycle Analysis of AA Alkaline Batteries - ScienceDirect.” Science Direct , 30 Apr. 2020, https://reader.elsevier.com/reader/sd/pii/S2351978920307794?token=83D8DF7FC82624070E11DF7A6D3ABE78563D7D5B907CE05FCDB7DC101ECAA5115D92567B9530C439B3577A7EBE50D2F0&originRegion=us-east-1&originCreation=20211103234732. Accessed 14 Nov. 2021
Holloway, James, et al. “China’s Steel Industry.” Reserve Bank of Australia , https://www.rba.gov.au/publications/bulletin/2010/dec/pdf/bu-1210-3.pdf . Accessed 12 Nov. 2021.
Moran, Luis, et al. “Electrical Energy Consumption Characterization of Open-Pit Mining and Mineral Processing Operations towards the Use of Renewable Energy Sources | IEEE Conference Publication | IEEE Xplore.” IEEE Xplore, Nov. 2019, https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=8911978. Accessed 14 Nov. 2021
The Editors of Encyclopaedia. “Electric Furnace.” Encyclopædia Britannica, Encyclopædia Britannica, https://www.britannica.com/technology/electric-furnace. Accessed 12 Nov. 2021.
“U.S. Energy Information Administration - EIA - Independent Statistics and Analysis.” Coal Is the Most-Used Electricity Generation Source in 18 States; Natural Gas in 16 - Today in Energy - U.S. Energy Information Administration (EIA), https://www.eia.gov/todayinenergy/detail.php?id=37034. Accessed 14 Nov. 2021
Marc Phan
Professor Cogdell
DES 40A
2 December 2021
Waste & Emissions of Disposable Batteries
While rechargeable batteries may be what make our modern life possible, there are still devices which take alkaline batteries in order to function. However, in order to get the battery into the device so that it can function, it must first be created and shipped to your area in order for you to even consider placing it into said device. Those processes from ore to battery and from mine to device are those that produce waste beyond the waste that is produced from creating the battery itself. Even then, while focusing on the waste that is produced from merely the production of the battery, that is ignoring the waste that comes at the end of the battery’s life, both from recycling it and even throwing it away in a trash can. This paper aims to focus on the entire life cycle of an alkaline battery, from start to finish, focusing on the wastes that are produced along the way, from its start inside the mines where its components are obtained, to the factories where they are assembled, on the ships which transport them to be consumed, within the devices that they power, and finally to the end of their lives, whether the material inside them is retrieved or thrown away whole.
Procuring the materials needed to produce batteries, particularly the material that conducts the electrical current, being the zinc and manganese dioxide, as well as a potassium solution (Florida State University), and the steel making up the exterior, is not a simple process, as they are located in different places, but that does not change the fact that sourcing these major components ends up producing waste. Starting with the metals, zinc, iron, and manganese, they are mined throughout various parts of the world, and to get to the ores, large amounts of material must be extracted, more specifically, on average, 1 ton of metal requires 22 tons of material to be mined (Office of Energy Efficiency & Renewable Energy), which is not also considering the water needed sometimes to effectively remove the metal from the rock and the chemicals that go to effectively obtaining more material (Geological Survey of Sweden). When not disposed of properly, these waste materials can cause damage to the environment and any settlements in close proximity (Zhang et al.;Monjezi et al.;Anju and Banerjee) due to the undesirable mineral-laced waste water leaking out into the ground and into the ecosystem. This also applies to the production of potash, which is converted into potassium, but because of its abundance in the Earth’s crust (Fleischer 2–3), the wastes are less intense than that of zinc. As can be seen, even when starting with raw materials, there is already a large amount of waste that is made when producing the ore for batteries, which is nothing to say of what is produced when refining and assembling the battery itself.
Once the raw ores are separated from the Earth, they must now be refined and assembled into a complete product ready to be shipped, which produces waste throughout. Starting with the raw ore, the metal must be pulverized in order to retrieve the metal compounds that then can be refined into the raw metal for battery production, and the main byproducts from this are called tailings (U.S. Environmental Protection Agency), which are in effect unwanted pulverized rock fragments mixed in with a large amount of water, and must be disposed of in a controlled manner, lest the toxic materials mixed within tailings, like sulfide materials (Schoenberger), leach out and acidify any nearby water sources and land it comes in contact with. Beyond that, however, now the concentrated metal compounds can be refined into their respective metals, and from the refining process a major byproduct is slag, the combined material which is not the metal desired at the end of the refining process, which has the potential to be repurposed into other applications depending on the origin of the slag (Piatak et al.). Now that the zinc, potassium, and manganese have been refined, all that’s left is the iron to be converted into steel for the battery’s exterior, a process which produces its own slag (Yi et al.) and requires refined coal, or coke, to produce, which means byproducts of coal are also included (American Iron and Steel Institute). Finally, now that the materials are present, the batteries can be assembled, and in assembling the battery, water must be used in various steps of the process to ensure the battery can adequately perform (US EPA). Now, with the battery being ready for shipping, there lies the problem with actually having to ship it to the consumer, who then can use it until the end of its charge.
Transporting the battery from wherever it is produced to the consumer, who then uses said battery to power their device, produces waste that isn’t necessarily from the battery itself. For example, if a battery needs to get from its factory in China to a store in the Midwestern US, it would need to hitch a ride on a cargo ship first, most of which use a heavy fuel oil, available for cheap, in order to power their movement, outputting many particularly nasty particulates such as sulphates and various metals into the air in the process, owing to the usually high impurity that is characteristic of heavy fuel oil, and that is not even including the additional carbon dioxide that is produced from the burning of the oil itself (Chu-Van et al.). However, even when it has finished its journey to the shore, there is still half a continent to cross before it reaches that store in the Midwest, which is where semi trucks come in to transport the finished battery the rest of the way there. However, while these semi trucks may be smaller than cargo ships, they are still a major contributor to climate change, accounting for a large slice of emissions, like carbon dioxide, sulphur dioxide, and carbon monoxide globally (Senarak). Still, once the battery reaches the intended target, that being your hands, there are still wastes even while using it, particularly what happens if they are left out for too long; if that manages to happen, potassium hydroxide, a white crystalline solid, will form on the negative side due to the battery leaking (Carl). When this happens, it’s probably a good time to consider the battery to be at the end of its life cycle and to pass it over to either a recycle center or simply throw them away if one wishes.
At the end of a battery’s life, two fates can befall it: it can go to a battery recycling center where the metal within can be retrieved to be used elsewhere or simply thrown away in the trash without much guilt. Why is that so? This is because, unlike some other batteries, the materials with more potential for environmental harm, zinc and manganese, don’t leak out of the battery like the potassium can, and thus don’t pose much threat to the environment surrounding it (Gasper et al.). Still, even though that much is true, that means the whole battery would then become a waste product, so recycling is still an option for the fate of your battery, and on that note, once the battery makes it to the recycling center, pyrometallurgy or hydrometallurgy can be used to either melt down the battery or draw out the desired metal via an aqueous solution reaction, with hydrometallurgy being particularly potent in extracting most of the zinc and manganese within a battery while only using sulphuric acid in order to draw the metals out of the battery, with the waste products being the sulfate, which can then be made into more acid to restart the process again (De Michelis et al.). Another method for retrieving battery metal is simply shredding the battery down and extracting the fragments out of the shreds, which is also effective in extracting the valuable metals from any alkaline battery (Gasper et al.). Even with these effective processes for extracting metal from batteries, the cost of these retrieval processes is not economically viable as of right now, and so landfilling batteries with little environmental impact seems to be a sensible option (Gasper et al.).
The life cycle of the alkaline battery from the mine to, eventually, the landfill, is a cycle that produces waste at each step, even through indirect means. From the raw ore straight out of the mines to the refinement of the ore into metal, which is then used to make batteries which are shipped to consumers, eventually either to be recycled or landfilled, all these steps produce waste at some step. One thing important to mention is the importance of petroleum and coal, which play a huge and pivotal role in powering the machines that do the work gathering ore or the furnaces that produce the metal (US Energy Information Administration). As the largest energy producer, these fossil fuels power the backbone of the life cycle and thus the wastes and emissions they produce are included as well. Besides that, alkaline batteries are just one example of how complex these life cycles of the things that we only ever see one step of really are, that massive amounts of energy go into each of these steps of producing commodities that we take for granted. It really makes one think, and possibly think of a different worldview, viewed through the lenses of the amount of energy everything around us takes to be made. It may be far from making the world a better place, but maybe that’s just step one of imagining a better life cycle for the whole world.
Works Cited
al Rawashdeh, Rami, and Philip Maxwell. “Analysing the World Potash Industry.” Resources Policy, vol. 41, Sept. 2014, pp. 143–151, 10.1016/j.resourpol.2014.05.004. Accessed 16 Oct. 2019.
American Iron and Steel Institute. “Steel Production.” American Iron and Steel Institute, www.steel.org/steel-technology/steel-production/.
Anju, M., and D. K. Banerjee. “Associations of Cadmium, Zinc, and Lead in Soils from a Lead and Zinc Mining Area as Studied by Single and Sequential Extractions.” Environmental Monitoring and Assessment, vol. 176, no. 1-4, 23 July 2010, pp. 67–85, 10.1007/s10661-010-1567-4. Accessed 26 May 2020.
Carl. “Is It Dangerous When Alkaline Battery Leaks? - Microcell Battery.” Https://www.micropower-Battery.com/, www.micropower-battery.com/is-it-dangerous-when-alkaline-battery-leaks/. Accessed 1 Dec. 2021.
Chu-Van, Thuy, et al. “On-Board Measurements of Particle and Gaseous Emissions from a Large Cargo Vessel at Different Operating Conditions.” Environmental Pollution, vol. 237, June 2018, pp. 832–841, 10.1016/j.envpol.2017.11.008. Accessed 4 Nov. 2021.
De Michelis, I., et al. “Recovery of Zinc and Manganese from Alkaline and Zinc-Carbon Spent Batteries.” Journal of Power Sources, vol. 172, no. 2, Oct. 2007, pp. 975–983, 10.1016/j.jpowsour.2007.04.092. Accessed 15 Nov. 2021.
Fleischer, Michael. Recent Estimates of the Abundances of the Elements in the Earth’s Crust. Washington, 1953, pp. 2–3.
Florida State University. “Molecular Expressions: Electricity and Magnetism: Alkaline Battery.” Micro.magnet.fsu.edu, 1995, micro.magnet.fsu.edu/electromag/electricity/batteries/alkaline.html.
Gasper, Paul, et al. “Economic Feasibility of a Mechanical Separation Process for Recycling Alkaline Batteries.” Journal of New Materials for Electrochemical Systems, vol. 16, no. 4, 4 Oct. 2013, pp. 297–304, 10.14447/jnmes.v16i4.157. Accessed 30 Nov. 2021.
Geological Survey of Sweden. “Chapter 4: Mining Waste.” Www.sgu.se, www.sgu.se/en/itp308/preparatory_course/4-mining-waste/.
Hamade, Ramsey, et al. “Life Cycle Analysis of AA Alkaline Batteries.” Procedia Manufacturing, vol. 43, 2020, pp. 415–422, 10.1016/j.promfg.2020.02.193.
Monjezi, M., et al. “Environmental Impact Assessment of Open Pit Mining in Iran.” Environmental Geology, vol. 58, no. 1, 26 Aug. 2008, pp. 205–216, link.springer.com/article/10.1007/s00254-008-1509-4, 10.1007/s00254-008-1509-4.
Office of Energy Efficiency & Renewable Energy. “Mining Industry Profile.” Energy.gov, www.energy.gov/eere/amo/mining-industry-profile.
Piatak, Nadine M., et al. “Characteristics and Environmental Aspects of Slag: A Review.” Applied Geochemistry, vol. 57, June 2015, pp. 236–266, 10.1016/j.apgeochem.2014.04.009. Accessed 3 July 2020.
Schoenberger, Erica. “Environmentally Sustainable Mining: The Case of Tailings Storage Facilities.” Resources Policy, vol. 49, Sept. 2016, pp. 119–128, 10.1016/j.resourpol.2016.04.009. Accessed 23 Oct. 2019.
U.S. Environmental Protection Agency. “Technical Report Design and Evaluation of Tailings Dams.” Web.archive.org, 10 May 2013, web.archive.org/web/20130510155552/epa.gov/waste/nonhaz/industrial/special/mining/techdocs/tailings.pdf. Accessed 1 Dec. 2021.
United States Environmental Protection Agency. Emission Facts Average In-Use Emissions from Heavy-Duty Trucks. Oct. 2008.
United States Geological Survey. “Zinc Statistics and Information.” Www.usgs.gov, www.usgs.gov/centers/nmic/zinc-statistics-and-information.
US Energy Information Administration. “Use of Energy Explained.” Eia.gov, 2016, www.eia.gov/energyexplained/use-of-energy/.
US EPA, OW. “Battery Manufacturing Effluent Guidelines.” Www.epa.gov, 6 June 2016, www.epa.gov/eg/battery-manufacturing-effluent-guidelines#what-is.
Yi, Huang, et al. “An Overview of Utilization of Steel Slag.” Procedia Environmental Sciences, vol. 16, 2012, pp. 791–801, www.sciencedirect.com/science/article/pii/S1878029612006469, 10.1016/j.proenv.2012.10.108.
Zhang, Xiuwu, et al. “Impacts of Lead/Zinc Mining and Smelting on the Environment and Human Health in China.” Environmental Monitoring and Assessment, vol. 184, no. 4, 14 May 2011, pp. 2261–2273, 10.1007/s10661-011-2115-6.