Design Life-Cycle
assess.design.(don't)consume
Brooke McDaniel
DES 40A – Section 2
Group 3 – with Briana Omori & Adrienne Lee
Topic: Dr. Martens 1490
4 December 2019
Materials Used in a Dr. Martens 1460 Boot
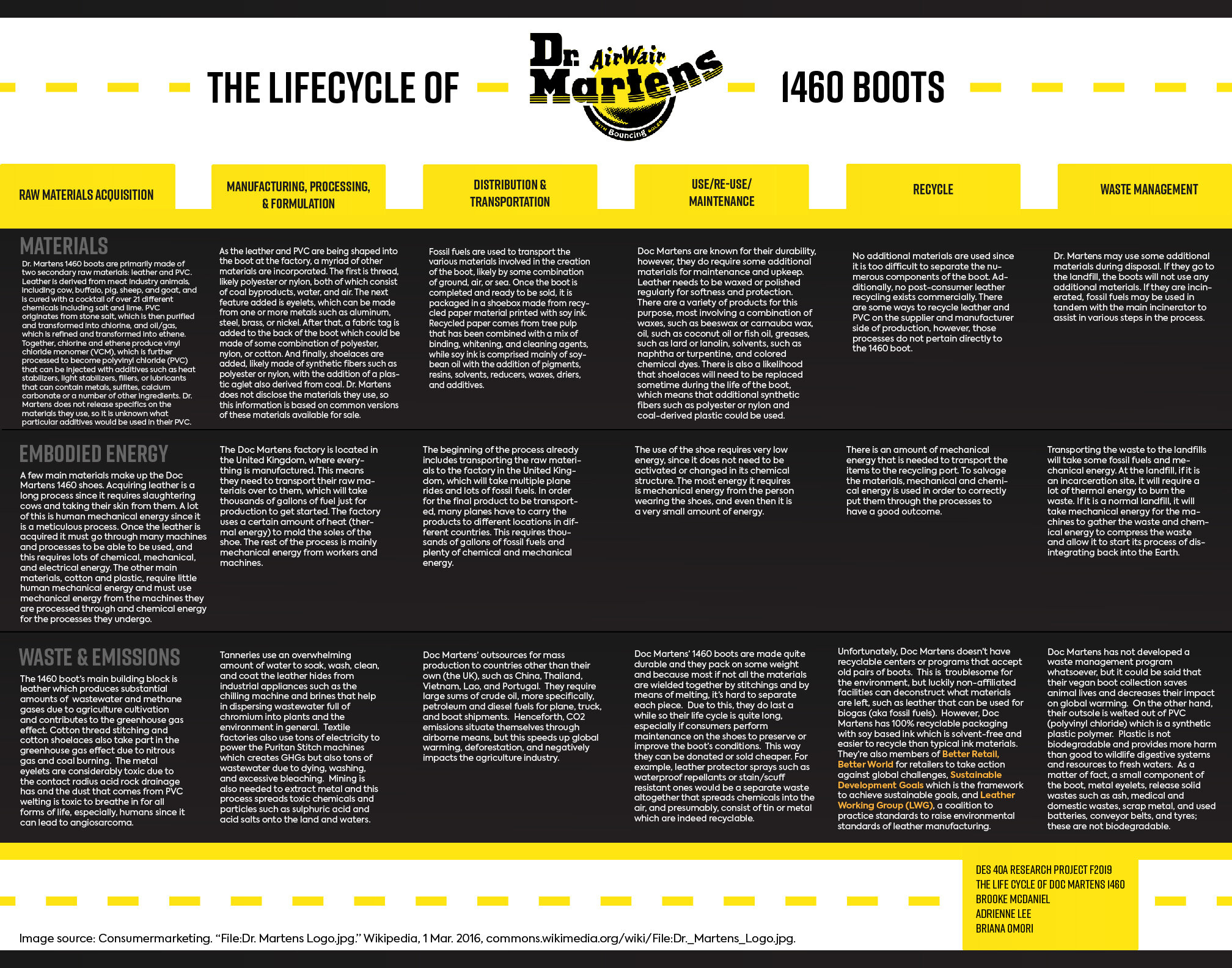
An iconic staple for punk culture and symbol of rebellion, Dr. Martens boots have been in production since the 1940s. Their comfortable “AirWair” rubber sole and renowned durability continue to draw customers from every generation, and create a demand for new and classic products. Durability in an era of cheap fast-fashion is notable, since consumers can feel good about buying a product that will not contribute to the growing pile of worn-out items in landfills. Additionally, Dr. Martens presents themselves as a socially-conscious company with publications such as the “Social Responsibility Initiative” on their website, which is valued image by the liberal audience they serve. However, the scope enumerated in this initiative is very narrow and the information errs on nontransparent for the overall lifecycle of their products. In particular, information on which materials the company uses and where they are sourced is scarce; only two secondary raw materials—leather and rubber—are ever cited by them. In order to better understand the impact of this company’s products, we have conducted independent research, focusing on a singular case study: the 1460 boot, which is a classic style that has been production since the 1960s. Our findings indicate that although only two materials are publicly cited by the company, the Doctor Martens 1460 boot combines innumerable natural, synthetic, and chemical raw materials that must undergo significant processing throughout the life cycle to create a finished product.
Dr. Martens 1460 boots are primarily made of two secondary raw materials: leather and PVC. Leather is derived from meat industry animals, including “cow, buffalo, pig, sheep, and goat” who are raised for food, not just for their hides (“Animal Derived Materials Policy”). The lifecycle of this material begins with agriculture, using seeds, dirt, fertilizers and pesticides to grow crops that are then made into feed and consumed by the animals. Once mature enough, the animals are slaughtered and separated into various parts including the hide, which must undergo a great deal of processing using a cocktail of chemicals. Tanning, an important phase in the process, alone uses over twenty-one different substances such as salt, lime, chromium sulphate, acids, aldehyde tanning agents, biocides, surfactants, and many others (“Chemicals Used in Leather Processing”). The full process—essentially preservation, tanning, and finishing—can use a total of 130 different chemicals (Chattha and Shaukat) derived from numerous raw forms, in addition to large amounts of water and coal-based energy (Joseph and Nithya 676). The result is a usable secondary raw material that can be further processed into a product with dying, trimming, and shaping. Dr. Martens has established certain standards that it follows in obtaining leather, mainly that their suppliers are certified by the Leather Working Group; however, the complexity of the process and the number of chemicals used still poses a significant concern for workers and the environment with the toxic wastes it produces.
The other main material used to construct the boot is Polyvinyl chloride (PVC), a plastic derived from a combination of chemicals. Dr. Martens commonly describes the sole of the shoe as being made of rubber with no other specifications, however, in a Business Insider video tour of their Newcastle factory Steve Bent, a production manager, describes the use of a PVC welt and sole and there are other sources that suggest PVC is used. Since there is no evidence for alternatives, it assumed that PVC is a primary material in the making of the 1460 boot. PVC consists of two primary raw materials: stone salt, which is then purified, combined with water, and converted by electrolysis into chlorine, and oil, which undergoes cracking and cooling to produce liquid ethylene. Together, chlorine and ethylene are combined to produce vinyl chloride monomer (VCM), which is further heated to become PVC resin. The resulting material can then be injected with additives such as plasticizers to add flexibility, fillers to add volume, heat stabilizers, light stabilizers, or lubricants that can contain metals, sulfites, calcium carbonate or a number of other ingredients. Dr. Martens does not release specifics on the materials they use, so it is unknown what particular additives would be used in their PVC. Once the PVC has been injected with any additional additives, it is shaped into small pellets that can then be melted and molded for use in the final 1460 boot.
As the leather and PVC are being shaped into the boot at the factory, a myriad of other materials are incorporated: thread, a fabric tag loop, meatal eyelets, shoe laces, and likely one or two other elements that are not immediately visible. Dr. Martens discloses very little information on these smaller elements, so this paper is relying on visual observation to identify these components initially and is basing the material information on common versions of these components available for sale online. The thread is likely polyester or nylon, both of which consist of petroleum byproducts, water, and air. Another option for thread is cotton coated in wax, however, this is a less likely option since synthetic materials are stronger, more durable, and work better with sewing machines by not leaving a residue. The next feature added is eyelets, which can be made from one or more metals such as aluminum, steel, brass, or nickel. After that, a fabric tag is added to the back of the boot which could be made of some combination of polyester, nylon, or cotton. And finally, shoelaces are added, likely made of synthetic fibers such as polyester or nylon, with the addition of a plastic aglet also derived from petroleum.
Beyond those seen in physical finished product, additional raw materials are used in transportation and distribution of the boot. Fossil fuels are used to transport the various materials involved in the creation of the boot throughout all stages of production, likely by some combination of ground, air, or sea. In their article “Material Flows in the Life Cycle of Leather,” Kurian Joseph, N. Nithya found that leather is transported “mainly by heavy-duty diesel truck” (678), however, tracking the raw materials involved in transportation beyond this point is not possible due to the diversity of the materials used, their complex production processes, and the extremely large scope of the supply chain. In addition to the materials for transportation, the boot uses other materials for packaging and distribution once fully formed and ready to be sold. The main packaging is a shoebox made from recycled paper material. Paper comes from tree pulp that has been combined with a mix of binding and whitening agents, and when it is recycled, new cleaning and whitening agents are added. These shoeboxes are printed with soy ink soy ink, a type of ink is comprised mainly of soybean oil with the addition of pigments, resins, solvents, reducers, waxes, driers, and additives (Trefil).
Once distributed to the consumer, Doc Martens are known for their durability, however, they do require some additional materials for maintenance and upkeep. Leather needs to be waxed or polished regularly for softness and protection. There are a variety of products for this purpose, most involving a combination of waxes, such as beeswax or carnauba wax, oil, such as coconut oil or fish oil, greases, such as lard or lanolin, solvents, such as naphtha or turpentine, and colored chemical dyes. There is also a likelihood that shoelaces will need to be replaced sometime during the life of the boot, which means that additional petroleum-derived synthetic fibers such as polyester or nylon and plastic could be used.
At the end of its lifecycle, the 1460 boot is disposed of and either put in a landfill or incinerated where some additional materials can be introduced. Recycling is not possible at this time since it is too difficult to separate the numerous components of the boot and no post-consumer leather recycling exists commercially. This means there are no new materials are used in recycling. Some ways to recycle leather and PVC on the supplier and manufacturer side of production exist and they use a variety of additional raw materials, however, those processes do not pertain directly to the product being examined. Dr. Martens boots may use some additional materials during disposal depending on the method. If they go to the landfill, the boots will not use any additional materials. If they are incinerated, fossil fuels may be used to assist the main incinerator with various steps in the process.
In all, the Dr. Martens 1460 boot uses of a vast variety of materials that must go through extensive processing. Although leather and PVC, the primary substances used to construct the boot, utilize some natural sources, they heavily rely on chemicals that are derived from numerous raw forms as well as water and electricity. During production of the boot, at least four more components are added, each a product of numerous and difficult to trace raw materials since Dr. Martens does not specify what they use. Throughout the production of materials and manufacturing of the product, fossil fuels are used for transportation, and once the boot is ready for distribution, additional materials are combined to create packaging. During use, the product continues to use new materials in the form of leather polish, made up of a variety of substances, and structural components such as shoelaces, which are also a product of various materials. When the boot has been used and is ready for disposal, it may use additional fossil fuels to be incinerated. Although Doc Martens are durable and long-lasting, the materials used in the 1460 boot demonstrate why consumers should be concerned about production processes in addition to product longevity if they want to do better for the earth. The raw materials consist mainly of chemicals and fossil fuels that produce toxic waste and affect workers and the environment. Dr. Martens does some work to mediate the impact of their material sourcing with the policies enumerated in their “Social Responsibility” publication, but there is a lot of work to be done if their products are to be fully socially and environmentally responsible. Finding ways to use fewer chemicals would reduce harmful wasted and finding ways to disassemble the boot after it is no longer usable would allow the PVC, a material made from a rapidly dwindling natural resource, to be reused.
Bibliography
“Animal Derived Materials Policy.” Dr. Martens, Dec. 2018. https://d3pjhixl6ywqix.cloudfront.net/product/uk-assets/corporate-and-press/drmartens-animal-derived-material-policy-2019.pdf. Accessed 24 Oct. 2019.
“Audit Protocols.” Leather Working Group. https://www.leatherworkinggroup.com/how-we-work/audit-protocols. Accessed 24 Oct. 2019.
Braun, Dietrich. "PVC—origin, growth, and future." Journal of Vinyl and Additive Technology 7.4 (2001): 168-176.
Braun, Dietrich. "Recycling of PVC." Progress in polymer science 27.10 (2002): 2171-2195.
Carey, Francis A. “Ethylene.” Encyclopaedia Britannica. https://www.britannica.com/science/ethylene. Accessed 4 Dec 2019.
Chattha, Jved Ahman, and M. Mobeen Shaukat. “An Assessment of Environmental Concerns in the Leather Industry and Proposed Remedies: A Case Study of Pakistan.” https://d3pcsg2wjq9izr.cloudfront.net/files/0/articles/2226/2045.pdf. Accessed 4 Dec 2019.
"Chemicals Used in Leather Processing – International School of Tanning Technology." Chemicals Used in Leather Processing – International School of Tanning Technology. https://sites.google.com/site/isttschool/useful-information/chemicals-used-in-leather-processing. Accessed 4 Dec 2019.
“Chlorine.” The Essential Chemical Industry – Online, 27 Nov 2018. http://www.essentialchemicalindustry.org/chemicals/chlorine.html. Accessed 4 Dec 2019.
Coxworth, Ben. “Recycling System Developed for Old Shoes.” New Atlas, 21 October 2013. https://newatlas.com/shoe-recycling-system/29479/. Accessed 4 Dec 2019.
Dixit, Sumita, et al. "Toxic hazards of leather industry and technologies to combat threat: a review." Journal of Cleaner Production 87 (2015): 39-49.
“Ethene (Ethylene).” The Essential Chemical Industry – Online, 4 Jan 2018. http://www.essentialchemicalindustry.org/chemicals/ethene.html. Accessed 4 Dec 2019.
Fela, Katarzyna, et al. "Present and prospective leather industry waste disposal." Polish Journal of Chemical Technology 13.3 (2011): 53-55.
“Freight Transport.” Wikipedia. https://en.wikipedia.org/wiki/Freight_transport. Accessed 4 Dec 2019.
Go, Jane and Trina Do. “Polyester.” Design Lifecycle, 13 March 2013. http://www.designlife-cycle.com/polyester. Accessed 4 Dec 2019. Accessed 4 Dec 2019.
“How is nylon made?” Open Learn, 26 Sept 2005. https://www.open.edu/openlearn/science-maths-technology/science/chemistry/how-nylon-made.
“How is Paper Recycled.” RecyclingGuide.org.uk. http://www.recycling-guide.org.uk/science-paper.html. Accessed 4 Dec 2019.
“How Is PVC Made, Anyway?” Teknor Apex, 31 March 2017. https://www.teknorapex.com/the-pvc-production-process. Accessed 4 Dec 2019.
“Incineration Processes and Environmental Releases.” National Center for Biotechnology Information, 2000. https://www.ncbi.nlm.nih.gov/books/NBK233627/. Accessed 4 Dec 2019.
Joseph, Kurian, and N. Nithya. "Material flows in the life cycle of leather." Journal of Cleaner Production 17.7 (2009): 676-682.
Karstadt, Myra. "PVC: health implications and production trends." Environmental health perspectives 17 (1976): 107-115.
Leadbitter, Jason. "PVC and sustainability." Progress in Polymer Science 27.10 (2002): 2197-2226.
“Leather Recycling.” Leather Sustainability. https://www.leathersustainability.com/is-the-recycling-of-leather-a-viable-option-for-brands-and-retailers/. Accessed 4 Dec 2019.
Litherland, Neal. “How Does a Waste Incinerator Work?.” Sciencing, 24 April 2017. https://sciencing.com/waste-incinerator-work-5245014.html. Accessed 4 Dec 2019.
Lopez, Leslie. “Leather Wastes and Emissions.” Design Lifecycle, 13 March 2014. http://www.designlife-cycle.com/leather. Accessed 4 Dec 2019.
Mad Madam Mel. “A recipe for Dubbin.” Mad Madam Mel, 27 Jan 2012. http://www.madmadammel.com/2012/01/recipe-for-dubbin.html. Accessed 4 Dec 2019.
McIntyre, J. Eric, ed. Synthetic fibres: nylon, polyester, acrylic, polyolefin. Taylor & Francis US, 2005.
“Modern Slavery and Transparency in the Supply Chain Statement.” Dr. Martens, 2018. https://d2g7c2xxqyt3nq.cloudfront.net/SocialResponsibility/ModernSlaveryAct.pdf. Accessed 24 Oct. 2019.
More evidence: Council, Northampton Borough. "Conservation Area Appraisal & Management Plan." (2011).
Nishihara, Toshio, and Tuyoshi Torikai. "Sewing thread for leather products and leather products produced by using the same." U.S. Patent No. 6,293,080. 25 Sep. 2001.
Northampton Borough Council. "Conservation Area Appraisal & Management Plan." (2011).
“Nylon.” New World Encyclopedia. https://www.newworldencyclopedia.org/entry/Nylon. Accessed 4 Dec 2019.
“Paper Recycling.” Waste Managemet. http://wmnorthwest.com/educational/paper.htm. Accessed 4 Dec 2019.
Peereboom, Eric Copius, et al. "Influence of inventory data sets on lifecycle assessment results: A case study on PVC." Journal of Industrial Ecology 2.3 (1998): 109-130.
“Polyvinyl Chloride.” Encyclopaedia Britannica, 28 Nov 2019. https://www.britannica.com/science/polyvinyl-chloride. Accessed 4 Dec 2019.
“Polyvinyl Chloride (PVC).” Lenntech. https://www.lenntech.com/polyvinyl-chloride-pvc.htm. Accessed 4 Dec 2019.
“Preparing PVC Pellets for Processing Environments.” Teknor Apex, 31 Aug 2017. https://www.teknorapex.com/pvc-pellets-for-processing-environments. Accessed 4 Dec 2019.
Raadschelders, Ellen, et al. "Side effects of categorized environmental measures and their implications for impact analysis." Environmental Science & Policy 6.2 (2003): 167-174.
Rahimifard, Shahin, Theodoros Staikos, and Gareth Coates. "Recycling of footwear products." Centre for Sustainable Manufacturing and Reuse/recycling Technologies (SMART), Loughborough University (2007).
Romeo, Claudia. “Inside Dr. Martens’ only UK factory where its iconic Made In England range has been manufactured since 1960.” Business Insider, 4 June 2018. https://www.businessinsider.com/dr-martens-uk-factory-cobbs-lane-where-iconic-made-in-england-range-is-made-2018-6. Accessed 25 Oct. 2019.
Sadat-Shojai, Mehdi, and Gholam-Reza Bakhshandeh. "Recycling of PVC wastes." Polymer degradation and stability 96.4 (2011): 404-415.
Saeki, Y., and T. Emura. "Technical progresses for PVC production." Progress in polymer science 27.10 (2002): 2055-2131.
Senthil, Rethinam, et al. "Recycling of finished leather wastes: a novel approach." Clean Technologies and Environmental Policy 17.1 (2015): 187-197.
“Shoe Care for Your Docs.” Dr. Martens. https://www.drmartens.com.au/post/shoecare-for-your-docs.html/. Accessed 4 Dec 2019.
Smith, James. “Dr. Martens – History, Philosophy, and Iconic Products.” Heddels, 27 Aug. 2018. https://www.heddels.com/2018/08/dr-martens-history-philosophy-iconic-products/. Accessed 24 Oct. 2019.
“Social Responsibility.” Dr. Martens, 2019. https://www.drmartens.com/us/en/social-responsibility. Accessed 24 Oct. 2019.
Sundar, V John, R Ramesh, PS Rao, P Saravanan, B Sridharnath and C Muralidharan. “Water Management in Leather Industry.” Journal of Scientivic & Industrial Research, June 2001. Vol. 60: 443-450.
“Thread.” How Products Are Made. http://www.madehow.com/Volume-5/Thread.html. Accessed 4 Dec 2019.
“Thread for Sewing Leather.” The Thread Exchange. https://www.thethreadexchange.com/miva/merchant.mvc?Screen=CTGY&Category_Code=leather-sewing-thread. Accessed 4 Dec 2019.
Titow, M. V. PVC technology. Springer Science & Business Media, 2012.
Trefil, Christopher. “Soy Based Inks Raw Materials.” Design Lifecycle, 13 March 2014. http://www.designlife-cycle.com/soy-based-inks. Accessed 4 Dec 2019.
Ullmann, Maryann. “Chemicals Used in Paper Recycling Mills.” BizFluent, 26 Sept 2017. https://bizfluent.com/facts-5731899-chemicals-used-paper-recycling-mills.html. Accessed 4 Dec 2019.
“What Goes Into Leather Polish?” Pure Polish, 16 April 2019. https://www.purepolishproducts.com/blogs/shoe-polish-and-leather-care-blog/what-goes-into-leather-polish. Accessed 4 Dec 2019.
“Which is made from a plant silk, linen or nylon?” UCSB ScienceLine, 10 July 2012. http://scienceline.ucsb.edu/getkey.php?key=3341. Accessed 24 Oct. 2019.
Wolfe, Isobella. “How Ethical Is Dr Martens?” Good on You, 17 Nov 2017. https://goodonyou.eco/how-ethical-dr-martens/. Accessed 24 Oct. 2019.
Adrienne Lee
Briana Omori, Brooke McDaniel
DES40A
Professor Cogdell
Embodied Energy of Doc Martens 1460
In a fast paced lifestyle that most people live in, fashion is ever evolving. One of the most consistent fashion items has been the Doc Martens 1460, a style of black leather boot that became a classic shoe over time. A shoe that will never go out of style will ask for a lot of the manufacturing and production industry, and will thus make its impact this way not only on society but on the world. The process of making Doc Martens 1460 uses many sources of energy from the very beginning of its life cycle to the end, and the final amount of embodied energy is calculated through the energy used in collecting raw materials such as leather, rubber, and fabric and putting them together, distributing and transporting the shoes, consumer use, and recycling and disposing of the shoe.
To start off the process, many raw materials have to be acquired in order to properly make the Doc Marten shoe. The main materials used are rubber for the sole of the shoe, leather for the base of the shoe, plastic for the rim of the shoelaces, and fabric/thread for the shoelaces. Rubber from its origins has been extracted naturally in order to be used, but there is less natural rubber available now. According to General Kinematic News, “A process called rubber tapping is used to harvest latex from rubber trees. A wide cut is made in a tree’s bark, allowing the latex to drip and be collected” (par. 3). Because the cut needs to be precise, most of the energy used for this process is human mechanical energy. After that, when the rubber is being washed and coagulated with acids, the energy is also human energy because these processes are hard to complete with only machines. The rubber is then transported, and since it is mostly made in Southeast Asia, transporting it by plane will take around 36,000 gallons of fuel, according to How Stuff Works (par. 1). Another material that goes into the soles is Polyvinyl Chloride (PVC), which is made through the chemical energy of combining chlorine and ethylene. This is combined with the rubber to create the sturdy sole of the shoe. Next comes the shoelaces, which require the extraction of cotton, polyester, nylon, and plastic (for the aglet) and spinning them until they form the shape of shoelaces. According to Barnhardt Cotton Production, the process of extracting cotton is mostly cleaning, purifying, and drying the cotton so that it is ready to be spun. The main methods of doing this are with machines and heat. Though there is no information on how much energy these machines use and how much heat is used, there is not a lot of human energy used in these processes besides feeding the cotton into the machines. One of the most important parts of the Doc Martens 1460 shoe is the leather that makes up most of the shoe. It is the most difficult to obtain and maintain, since according to Leather Dictionary, leather “undergoes between 35 to 55 different processes” in order to be ready to be shipped out and manufactured (par. 1). The first few processes require only human energy, since it is more meticulous - cutting the raw hide, then soaking it in salt water to cure it and liming it to separate the hairs from the hides and to clean it and prep it for the next steps. Further cleaning and stripping the leather of its fats and acids are done using machines and a little bit of help from human workers. The rest of the process is done using machines in a factory. The largest leather manufacturer is China, and it generally uses coal to fuel its textile factories. China has a history of using over 2.5 billion pounds of coal for its textile factories, so one can imagine that a fraction of that is used to fuel the leather factories. Lastly, plastic is needed for the shoelace aglets and the eyelets for the shoelace holes on the shoe. This process begins in an oil refinery, where crude oil is mixed with a catalyst to create a reaction that ultimately leads to the creation of plastic (Plastics Europe). This is mostly done by machines, so the energy used here is mechanical. Once the plastic is shaped and cut to the correct shape, it is ready to go. Some human mechanical energy must be used here to collect the plastic all together. Though the raw materials have some processing to go through in order to be extracted, there are more processes for them to go through once manufacturing begins.
Manufacturing of the shoe itself requires machines that need to use a higher amount of power and fuel. Once the raw materials are acquired and ready to use, they are sent to the United Kingdom, where the Doc Martens factory is located. On the Doc Martens Modern Slavery and Transparency in the Supply Chain Statement, Doc Martens lists Asia, Europe, and South America as the locations of their biggest manufacturers (page 5). To transport the materials from those areas to the United Kingdom, fossil fuels will be used to fuel the planes that will be taking them across. A plane from Asia to Europe will take approximately 4,000 miles and a plane typically takes 5 gallons of fuel per mile, so transporting materials from Asia to the UK will take around 20,000 gallons of fuel. By using the same approximation, it will take around 6,000 miles from South America to Europe; therefore, it will take around 30,000 gallons of fuel for that trip. Lastly, transporting items across Europe has a large range but to approximate a 2,000 mile trip will take around 10,000 gallons of fuel. At the Doc Martens factory, all the shoes are put together and packed to be sold in stores all around the world. Though at this point all the raw materials have been gathered, they are not necessarily in prime shape to go straight into production. Once the raw materials arrive, there is a group of workers that will assign each material to its station to be cut and styled and put together to complete the shoe. The leather will have to be dyed and checked to make sure it will match the Doc Martens style, and this will require mechanical energy from the machines. A lot of the cutting is done by the employees with the help of electronic machines, which will use mostly mechanical energy. Since the sewing of the leather to the other parts will require more attention, it is not completely done by machines but rather assisted by employees.The shoelaces will also have to be spun into shape and blended with the different colored threads, which will be dyed in the factory. This uses a lot of mechanical and chemical energy since the thread needs to be put through a process that will change their appearance. A machine is used to spin the shoelaces in place and turn them into the classic Doc Marten round and smooth shoelace. The soles of the shoes will also have to be moulded by using heat and machine molds. The main material the soles are made of is rubber, and the melting point of rubber is approximately 180 degrees celsius. To get a furnace to that temperature, it will not necessarily take a lot of coal (approximately 40 pounds) but it takes a few hours to let it burn to a temperature high enough. This process uses a bit more thermal energy than the rest, but it still requires mechanical energy from the machines and the workers. All the details are finished off with the workers, and the shoe is ready to be packed and shipped off to other countries. Once the product is out in stores and going, the consumer will eventually out wear the shoe and want to recycle it. Then, there is another energy process that goes completely through the life cycle.
After the product is finished and is in the hands of its consumer, the consumer will wear it until it needs to be recycled. There is not a lot of energy that goes into the use of the shoe, since it is a fashion item and it is worn with mostly mechanical energy. It requires nothing to be done to it in order to be used, so it has low energy usage. Thus, the processes other than production that will take up a lot of energy is recycling and putting the shoe to waste. The shoebox is the one part of the product that is “not only fully recyclable but is made from 100% recycled materials, and it is printed with soy ink, which is solvent-free and easier to recycle than traditional inks” according to the official Doc Martens site. The site also states that any customer can send their shoes back to their warehouse in the United Kingdom to be recycled. That is one option along with taking them to thrift stores or any textile recycling port. This would take the energy of the customer and the vehicle they are taking to these places along with the fuel it takes for the recycler to take them to the factory to go through the recycle process. Since leather is a big part of the shoe, leather might be the only part of the shoe that can be recycled and reused. However, according to Business Recycling, “in order to make leather workable and durable for use in a range of products it must go through a tanning process” (par. 2). The tanning process is long and takes a lot of water and toxic chemicals to complete it. The turnaround for leather is also small, since not all parts of the leather end up being able to be recycled. Though there is no information on the exact amount of water used for this process, there is for sure the presence of chemical and mechanical energy. Though some materials can be saved by recycling, the other materials which are unable to be used are put into the dumpster.
The waste industry had its own ways of doing things, and there are generally designated areas where dumpsters are located. With this comes the issue of transporting all the materials to the waste site along with the energy the dumpster uses to organize and rid of all the trash. According to Arthur Murray, “For every 100 pounds of municipal solid waste in the U.S., more than 85 pounds can be burned, releasing heat that can turn water into steam that can turn a turbine to generate electricity” (Dumpster.com, par. 12). This means that most dumpsters use thermal and chemical energy, because it needs to heat up the trash hand turn the steam into power. The incarcerators are heated up to 1,800 degrees fahrenheit, which means it will take hundreds of pounds of coal or fuel in order to get to that temperature. In other landfills where incarceration is not used, machines use mechanical energy to line up the trash and compress it so that it will decompose into the soil. This is a long process, however, and will take small amounts of chemical energy throughout a long period of time. The complete embodied energy to put the Doc Martens shoes to waste must include the transportation of the waste items to the actual landfill, which requires not only the owner of the shoes to either drive to their nearest dumpster or have a dump truck collect the trash from outside their front door. After that, the trucks must transport the items to the landfill site, which might be several miles away. Not every city has its own local landfill that is able to incarcerate items and distribute them evenly for proper disintegration into the Earth. Though disposing of the shoes seems like a simple task, it actually requires a lot of different types of energy and can put a lot of stress onto our planet.
In the current world we live in now, the main source of energy that is used in the production of fashion and other products is machines. These machines are powered by electricity or fuel, and rarely use human energy to make them. Because they need to be made at a fast pace, it is hard for the product to be made with anything other than machines. They are more efficient and can be cleaner, and it is easier to regulate them because they will do whatever they are programmed to do. One form of energy that has stayed constant throughout all the processes is the use of fossil fuels. Petroleum gas is used in planes to transport the raw materials to the Doc Martens factory and the finished products to store locations all across the world. The making of the Dr. Marten 1460 shoes requires the mechanical energy of machines and humans as well as thermal energy for part of the production and fossil fuels to transport the product from place to place.
Bibliography
“How Is China's Energy Footprint Changing?” ChinaPower Project, 13 Aug. 2018,
chinapower.csis.org/energy-footprint/. Accessed 24 Nov. 2019.
“How Shoelaces Are Made.” How Shoelaces Are Made.,
fabmania.com/how_shoelaces_are_made.htm. Accessed 25 Oct. 2019.
HowStuffWorks.com. “How Much Fuel Does an International Plane Use for a
Trip?” HowStuffWorks Science, HowStuffWorks, 28 Jan. 2015,
science.howstuffworks.com/transport/flight/modern/question192.htm. Accessed 24 Nov. 2019.
IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. “Occupational
Exposures in the Rubber-Manufacturing Industry.” Chemical Agents and Related
Occupations., U.S. National Library of Medicine, 1 Jan. 1970, www.ncbi.nlm.nih.gov/books/NBK304412/. Accessed 25 Oct. 2019.
Jr, Esteban Robles, et al. “How Is Polyester Made? - How Is Polyester Made?” Craftech
Industries, 14 Nov. 2019, www.craftechind.com/how-is-polyester-made/. Accessed 28
Nov 2019.
Lazaroiu, Gheorghe, et al. “Solutions for Energy Recovery of Animal Waste from Leather
Industry.” Energy Conversion and Management, vol. 149, 2017, pp. 1085–1095., doi:10.1016/j.enconman.2017.06.042. Accessed 30 Nov. 2019.
“Leather.” Business Recycling, businessrecycling.com.au/recycle/leather. Accessed 26 Nov.
2019.
“Leather Industry.” Leather Industry - an Overview | ScienceDirect Topics,
www.sciencedirect.com/topics/earth-and-planetary-sciences/leather-industry. Accessed
24 Oct. 2019.
“Leather Production.” Dictionary.com,
www.leather-dictionary.com/index.php/Leather_production. Accessed 25 Oct. 2019.
“Modern Slavery and Transparency in the Supply Chain Statement.” Dr. Martens, 2018.
https://d2g7c2xxqyt3nq.cloudfront.net/SocialResponsibility/ModernSlaveryAct.pdf.
Accessed 24 Oct. 2019.
Muñoz, Zayetzi Rivera. Water, Energy and Carbon Footprints of a Pair of Leather Shoes. ITM
School of Industrial Engineering and Management. KTH Royal Institute of Technology,
2013. Accessed 24 Oct. 2019.
“Raw Cotton Processing: How Is Cotton Processed: Barnhardt Cotton.” Barnhardt Purified
Cotton, www.barnhardtcotton.net/technology/cotton-processing/. Accessed 28 Nov.
2019.
“Shoelace.” How Products Are Made, www.madehow.com/Volume-6/Shoelace.html
Accessed 25 Oct. 2019.
“Social Responsibility.” Dr. Martens, 2019.
https://www.drmartens.com/us/en/social-responsibility. Accessed 24 Oct. 2019.
“The Life Cycle of Rubber.” General Kinematics, General Kinematics, 20 Feb.
2019, www.generalkinematics.com/blog/the-life-cycle-of-rubber/. Accessed 25 Oct. 2019.
“Think Twice Before You Throw Out Your Plastic Water Bottle.” Get the Facts About Trash
Production in the U.S. | Dumpsters.com, www.dumpsters.com/blog/us-trash-production.
Accessed 30 Nov. 2019.
“Trader Protocol 2.0 Is Launched.” Improving Environmental Stewardship,
www.leatherworkinggroup.com/how-we-work/audit-protocols/trader-protocol. Accessed
24 Oct. 2019.
“Where Does Our Trash and Garbage Go?: Budget Dumpster.” Where Does Our Trash and
Garbage Go? | Budget Dumpster,
www.budgetdumpster.com/resources/where-does-trash-go.php. Accessed 29 Nov. 2019.
Briana Omori
Professor Cogdell
DES 40A
26 Nov 2019
Waste and Pollution: If the Boot Fits, Wear the Doc Martens 1460 Smooth
The Northamptonshire, English footwear and clothing company, Dr. Martens, originally designed their shoes for better fit and comfort for the middle class workforce; however, they became incredibly popular among youth culture during the 1960s which was “culturally rebranded” for its edgy, self-expressive style. With such a huge reputation to their name, supply and demand continues to skyrocket; as of August 2019, the company reported “...its fiscal 2019 sales results this week, noting a 30% climb in revenues to 454.4 million pounds (US $547.9 million) and a 70% leap in profits to 85 million pounds (US $102.5 million) for the 12 months ended March 31” (Bell). In accordance, the CEO of Doc Martens, Kenny Wilson, hopes to continue increasing productivity rates and sales. In the year 2020, Doc Martens will celebrate the 60th anniversary of their most iconic boot collection, the 1460 Smooth, by launching a new limited-edition collaboration every month (Bell). Despite commercial benefits, Doc Martens’ use of manufacturing through outsourcing is a huge negative for the agricultural industry and fuels deforestation on land, increases greenhouse gases within the atmosphere, and expands toxic chemicals, fumes, and dyes within fresh waters.
Leather is the primary building block for the production of Doc Martens’ 1460 Smooth and is also a byproduct of the meat industry. Doc Martens’ stated, “In 2018 99% of all of our upper leather was sourced from LWG medal status tanneries” and that they do not use animal fur, species listed on CITES or IUCN list of endangered species, and leather or skin products from exotic or wild caught animals such as alligators, crocodiles, lizards, snakes and reptile skins (Social Responsibility). This is great news, but this means a majority of their leather is from the slaughtering of bovine. Leather is fabricated in tanneries through a lengthy process: preparation, beamhousing, tanning, and finishing. Throughout each process, exceeding amounts of water are used to soak, clean, or removal hair, dirt, or excess particles through lengthy processes that can take up to 8-24 hours. Chilling machines of various temperatures and fleshing machines wash or clean excess materials from the hide, brine baths of salt and vats full of cold water soak the hides, large rotating drums full of chromium salt water convert hides to leather, and flow coaters distribute dyes for colored boots (Leather). This heavy use of fresh water affects its accessibility for all living beings, especially agricultural farmers. It’s estimated “...besides the 30-35 m3 disposed to environment during the processing of every I ton of rawhide in world leather industry the data from FAO reveals that approximately 8.5 million tons of solid waste is generated during the production of 11 million tons of rawhide processed (Ozgunay 867). This solid waste has now since shot up to more than 600,000 lbs. with a loss of hides up to 40-50% due to shavings and trimmings (Zafar). According to Table I, solid wastes include untanned waste such as shavings, subepidermal, and tissue trimmings, tanned wastes such as shavings and split, and dyed and finished waste such as shavings and fluff. As a result, toxic fumes and chemicals such as chromium, sulphur, oils, and noxious gases such as ammonia, hydrogen sulphide are dispersed (Zafar); most notably, the burning of fossil fuels and heavy production of methane gases accelerate greenhouse effects affecting agriculture. In fact, “[g]lobally, agriculture contributes 65-80% of total N2O, mainly from nitrogenous fertilizers on cultivated soils, cattle and feedlots” (Khanal). With the need to access bovine, more area for cultivation is also needed which increases carbon dioxide levels, leading to global warming and deforestation. With such devastating effects on all three levels, the lifecycle of leather still proves to be the most lethal.
Doc Martens’ shoe laces add security between foot and boot and consist of two components: cotton woven tape to create the elastic stretch to tighten shoes and a plastic aglet for lacing into the eight individual metal eyelets. They are first created with electric motors of braiding machines which are then transferred to be coated with acetone by tipping machines and acetate tape by dies, heated and pressurized to seal the coatings, cut, placed together by a pairing machine, and packaged with blister packaging machines (Shoelace). Although simple in appearance, shoelace manufacturing requires many steps; this is a huge health hazard for our waters due to excessive bleaching and dying. In support of this, as of 2018, “The water used to make cotton is 60% of the total water footprint of fabric processing. This considerably outweighs its 43% share of the market” (Department for Environment). To add, these processes allows for “...liquid effluvia ejected from their factory and reclaim the water” (Shoelace). More importantly, the cotton in shoelaces requires consistent levels of nitrogen to create the synthetic fertilizer that it needs to grow which is hazardous to air quality; moreover, “[c]otton plants can only use so much nitrogen at a given time, and the excess is lost to water or air. It literally becomes money down the drain – and a potent pollutant. Nitrogen fertilizers are a major source of greenhouse gas emissions, particularly nitrous oxide” (Cotton Carbon Footprint). Despite being a ball of fluff that appears harmless, cotton leaves a carbon footprint and other harmful wastes.
Cotton’s detrimental effects within the atmosphere and waters also apply to their famous yellow stitching created in textile factories with a Puritan Stitch Machine. This machine runs on power done by animate prime movers through a pneumatic presser foot life system or inanimately with drive motors powered by voltages and frequencies installed (Industries). It is also capable of 1800 stitches per minute (Industries); whereas, industrial sewing machines operate at high speeds of 10,000 stitches per minute (Mellero). Regrettably, I couldn’t find facts on specific wastes or emissions produced from this particular machine. With Puritan Stitch machines not being as powerful as industrial machines, they require more time to get the job completed. It can be assumed that with electricity, a secondary source, coal production increases hence playing a part in greenhouse gas production.
Although a tiny component, Doc Martens’ punched metal eyelets hold laces together and comprise of two parts: the trumpet portion and the washer portion. First, holes are punched out in the material (in this case, leather), and trumpet and washer are crimped together with a hammer or with a specialized crimping tool to create the unified eyelet (About Eyelets). Although the process is quite simple, the extraction of metal is more burdensome with origins in the Earth’s core (IMS); therefore, mining is required. One of the most negatively pervasive effects from mining is acid rock drainage since this exposes the land, air, and waters to sulphuric acid and acid salts; other profound issues are in unstable water balances, the generation of dust and combustion of petroleum and diesel that release greenhouse gases into the atmosphere, and solid wastes such as ash, medical and domestic wastes, scrap metal, and used batteries, conveyor belts, and tyres (Spitz and John Trudinger). Although small, it packs a punch and requires the use of third-party suppliers!
To create the shape of the famous boot, Doc Martens’ uses PVC welting known as polyvinyl chloride which is a synthetic plastic polymer made of oil and salt to create the boot’s mould and outsole. Thermal cracking is used to separate ethylene out of the oil feedstock, then a process called electrolysis extracts chlorine out of sea salt, and then it goes through thermal cracking one last time (Teknorapex). Sad to say, PVC is difficult to recycle and fuels adverse effects within the water, air, and land, including affecting PVC facility workers’ health (Alessandro). Since it contains residue from different substances, exposure to it, most notably, PVC dust, has resulted in a rare cancer within the liver called angiosarcoma in workers (Karstadt); adding on, “exposure to PVC dust may cause pulmonary dysfunctions” (Karstadt). On the other spectrum, it can interfere with animals development and their productive systems (Alessandro). Looking back on the disastrous effects all of these materials, especially PVC, sustainable action needs to occur to save any resources that are left, but first and furthermost, what is one of the most vital resources that fuels these industries?
With outsourcing, the global transportation of products and materials expend an inordinate quantity of fossil fuels from trains, planes, and ships by suppliers outside of their UK headquarters such as China, Thailand, Vietnam, Lao, and Portugal. To meet the needs of consumers, Doc Martens has not opted for alternative energy resources to decrease their fossil fuel intake and output levels. Petroleum, derived from crude oil, is a non-renewable energy resource that takes millions of years to replenish, and because of increasing populations, the reserve supplies are being depleted at a quicker rate than ever before. In order to extract crude oil, it must go through hydraulic fracking. It is stated, “[t]he transport sector will be increasingly important as a consumer of oil, its share of final energy consumption of oil rising from 47% in 2002 to 54% by 2030” (Jolley). Negative effects are “stress on surface water and groundwater supplies from the withdrawal of large volumes of water used in drilling and hydraulic fracturing, contamination of underground sources of drinking water and surface waters resulting from spills and faulty well construction, adverse impacts from discharges into surface waters or from disposal into underground injection wells, air pollution from the release of volatile organic compounds, [and] hazardous air pollutants and greenhouse gases” (Lichtarowicz). Doc Martens has created initiatives to combat other issues.
Doc Martens’ 1460 boots uses make them durable and dense because due to the wielding of stitchings and melting making it hard to separate each piece; therefore, the life cycle is quite long, especially if consumers preserve or improve the boot’s conditions; this also means they can be donated or sold cheaper to be reused. For example, leather protector sprays such as waterproof repellants or stain/scuff resistant ones would be a separate waste altogether that spreads chemicals into the air, and presumably, consist of tin or metal which are indeed recyclable. Being more sustainable, their shoebox packaging is 100% recyclable with its prints being of a soy based ink that is solvent-free and easier to recycle than typical inks. More importantly, they released vegan boot collections, including a vegan 1460 line! In fact, they received the BREEAM Very Good award for being the top 25% for environmental performance for non-domestic UK structures’ sustainable design. They’ve installed solar thermal panels, rainwater harvesting, energy efficient LED lighting and air conditioning, and DC being zero waste. They’re also members of Better Retail, Better World for retailers to take action against global challenges, Sustainable Development Goals, a framework to achieve sustainable goals, and Leather Working Group (LWG), a coalition to practice standards to raise environmental standards of leather manufacturing. Sadly, Doc Martens doesn’t have recycling programs or centers to dispose of boots and there isn’t disclosure of what materials can be reused. Though, if stripped of certain parts, like the leather used, it can be purposed as biogas (Zafar). What does this mean for the future?
Doc Martens has taken leaps for sustainable design; however, a majority of their top sellers remain non-vegan. They’ve made progress towards a green working environment, but there is still much to be done on how green the production process is and how conscious they are of their “suspiciously” non-vegan sourced materials. A solution is greater advertisement of the vegan collection and the benefits it can provide for both consumer and distributor. If Doc Martens hosted recycling centers, raw materials could be reused for a “new” recycled boot collection; this will provide greater transparency and will reinforce their sustainable ambitions subtly but in an effective manner. It can be said that Doc Marten strives for a strong relationship with consumers and employees, and their next step is to make progress in the industrial spectrum.
Works Cited
Andrews, Texiera, et al. “Leather - Design Life.” Design Life-Cycle, Mar. 2014, www.designlife-cycle.com/leather.
Bosnic, M, et al. “POLLUTANTS IN TANNERY EFFLUENTS.” Leather Panel, 9 Aug. 2000, Leatherpanel.org.
Chen, Hualin, et al. “Mobility and Storage Sinks for Chromium and Other Metals in Soils
Impacted by Leather Tannery Wastes.” Journal of Environmental Monitoring, The Royal Society of Chemistry, 14 Nov. 2012, pubs.rsc.org/en/content/articlelanding/2012/EM/c2em30452j#!divAbstract.
Escoto Palacios, Maria Jose, et al. “From Leather Waste to Functional Leather.” From Leather Waste to Functional Leather, Oct. 2016, www.researchgate.net/publication/324390477_From_leather_waste_to_functional_leather.
“FAQ's: Leather Working Group.” Improving Environmental Stewardship, www.leatherworkinggroup.com/how-we-work/faqs.
“How Sustainable Is Dr. Martens ?” D,rankabrand.org/sustainable-shoes-footwear/Dr.+Martens.
Jeff. “Doc Martens: England vs. Thailand vs. China.” ALPHABET CITY, 1 Jan. 1970, www.alphabetcityblog.com/2009/05/doc-martens-made-in-england-vs-made-In.html.
“LWG Environmental Audit Protocol Responses Report Issue 6.6.2.” Leather Working
Group, 2019, www.leatherworkinggroup.com/contentfiles/LWG-775.pdf?v=1.
“Regulatory Information by Topic: Waste.” EPA, Environmental Protection Agency, 26 Mar. 2019, www.epa.gov/regulatory-information-topic/regulatory-information-
topic-waste#solid.
“Regulatory Information by Topic: Water.” EPA, Environmental Protection Agency, 22 Aug. 2019, www.epa.gov/regulatory-information-topic/regulatory-information-Topic-water.
Śmiechowski, Krzysztof, and Marzanna Lament. “Impact of Corporate Social Responsibility (CSR) Reporting on pro-Ecological Actions of Tanneries.” Journal of Cleaner Production, Elsevier, 23 May 2017, www.sciencedirect.com/science/article/pii/
S0959652617310442?via%3Dihub.
“Tannery Operations.” :: WorstPolluted.org : Projects Reports, www.worstpolluted.org/projects_reports/display/88.
Teng, Xu, et al. “Characteristics of Typical Pollutants in Tannery Site Soil - 75.” Zur Startseite, 26 June 2019, slub.qucosa.de/landing-page/?tx_dlf%5Bid%5D=http%3A%2F%2FSlub.qucosa.de%2Fapi%2Fqucosa%253A34271%2Fmets%2F&cHash=0c14fe8f2223fefc27e6f39f4e67d2a.
Wah, Samantha, et al. “Vans Old Skool Canvas Shoes - Design Life.” Design Life-Cycle, Dec. 2018, www.designlife-cycle.com/vans-old-skool?rq=outsole.
Wolfe, Isobella. “How Ethical Is Dr Martens?” Good On You, 7 Feb. 2019, goodonyou.eco/how-ethical-dr-martens/.
Bibliography
“About Eyelets.” About Eyelets, www.thomasnet.com/about/eyelets-26902403.html.
Alessandro, Nicole. “Why You Should Avoid PVC Products.” EcoWatch, EcoWatch, 18 June 2014, www.ecowatch.com/why-you-should-avoid-pvc-products-1881927242.html.
Bell, Jennie. “Dr. Martens CEO Reveals Why the Brand Is Growing - And It's Not Just the '90s Craze.” Footwear News, Footwear News, 15 Aug. 2019, footwearnews.com/2019/business/financial-news/dr-martens-business-strategy-dtc-stores-digital-kenny-wilson-1202816870/.
Buekens, Alfons, and Kefa Cen. “Waste Incineration, PVC, and Dioxins.” ProQuest, 28 Oct. 2010, search.proquest.com/docview/897348186?rfr_id=info%3Axri%2Fsid%3Aprimo.
“Cotton Carbon Footprint U.S.: Cotton LEADS: Cotton & Climate Change.” Cotton LEADS - Sustainable Cotton Production, cottonleads.org/sustainable-production/carbon-footprint-united-states/.
“Dr. Martens: A History of Rebellious Self-Expression.” Dr. Martens Official, www.drmartens.com/us/en/history.
Department for Environment, Food & Rural Affairs. “Resources and Waste Strategy for England.” GOV.UK, GOV.UK, 18 Dec. 2018, www.gov.uk/government/publications/resources-and-waste-strategy-for-england.
“Environmental Hazards of Leather.” PETA, 22 June 2010, www.peta.org/issues/animals-used-for-clothing/leather-industry/leather-environmental-hazards/.
Industries, Puritan. “Puritan Machine Features.” Puritan Machine Features, www.puritanindustries.net/features.htm.
IMS. “Where Do Metals Come From?” Industrial Metal Supply Blog, 10 Dec. 2018, www.industrialmetalsupply.com/blog/where-do-metals-come-from/.
Jolley, Ainsley. “The Supply of Fossil Fuels.” CORE, Mar. 2006, core.ac.uk/reader/10826325.
Karstadt, Myra. “PVC: Health Implications and Production Trends.” Environmental Health Perspectives, U.S. National Library of Medicine, Oct. 1976, www.ncbi.nlm.nih.gov/pmc/articles/PMC1475246/.
Khanal, R. “Climate Change and Organic Agriculture”. Journal of Agriculture and Environment, Vol. 10, Aug. 2009, pp. 116-27, doi:10.3126/aej.v10i0.2136.
Lichtarowicz, Marek. “Extracting Crude Oil and Natural Gas.” Essential Chemical Industry Online, 7 Sept. 2018, www.essentialchemicalindustry.org/processes/extracting-oil-and-natural-gas-fracking.html.
Martens, Dr. “DR. MARTENS MADE IN ENGLAND.” Dr. Martens Blog, 17 Jan. 2019, blog.drmartens.com/dr-martens-made-england/.
Mellero, Patricia, et al. “Monitoring and Control of Industrial Sewing Machines- Research on Thread Tension Behavior in Lockstitch Machines.” CORE, IEEE, 29 June 2017, core.ac.uk/display/132797756?recSetID=.
“Modern Slavery and Transparency In the Supply Chain Statement .” Dr. Martens: CA Supply Chains Act, 2018, d2g7c2xxqyt3nq.cloudfront.net/SocialResponsibility/ModernSlaveryAct.pdf.
Ozgunay, H, et al. “Characterization of Leather Industry Wastes.” https://www.researchgate.net/Publication/281414683_Characterization_of_leather_industry_wastes, Jan. 2007, www.researchgate.net/publication/281414683_Characterization_of_leather_industry_wastes.
“Patent Leather.” How Products Are Made, www.madehow.com/Volume-6/Patent-Leather.html.
“Shoelace.” How Products Are Made, www.madehow.com/Volume-6/Shoelace.html.
“Social Responsibility: Dr Martens Official Site.” Social Responsibility | Dr Martens Official Site, www.drmartens.com/uk/en_gb/social-responsibility.
Teknorapex. “Blogs.” The-PVC-Production-Process, 31 Mar. 2017, www.teknorapex.com/the-pvc-production-process.
Zafar, Salman. “Tannery Waste Management.” BioEnergy Consult, 12 Nov. 2019, www.bioenergyconsult.com/tag/tannery-waste-management/.
“16.2.4. Other Wastes.” Mining and the Environment: from Ore to Metal, by Karlheinz Spitz and John Trudinger, CRC Press/Balkema, Taylor Et Francis Group, 2019, pp. 468–477.