Design Life-Cycle
assess.design.(don't)consume
Gayatri Narayan
DES 40A Fall 2016
Professor Christina Cogdell, TA Alex Webster
Research Paper: Raw Materials
The Complexity of a Kiss: Investigating the Raw Materials of Hershey’s Packaging
Packaging is a vital section of the food industry worldwide, and is something most of us encounter yet overlook on a daily basis. When carefully considering the array of raw materials, energy, and waste emissions that are involved in the production, usage, and disposal of the packaging, it becomes apparent that it is not merely a covering for the product it carries- it is in fact a multiform product in itself. In this paper I will address the complexities of the packaging of a Hershey’s Kiss by conducting a detailed investigation of the raw materials required to produce the three main components of its wrapper; through this I hope to illuminate the intricacies of the life cycle of a seemingly simple product.
The most essential and integral portion of the packaging of each Hershey’s kiss is the aluminum foil used to wrap the distinctive flat-bottomed tear drop shaped chocolate. Standard household foil has a thickness of .00063 inches, whereas the lightweight and ultra-thin foil used to wrap the kisses is only .00035 inches thick (Maria). Aluminum foil is a very popular packaging material in the food industry because it is inexpensive, durable, non-toxic, as well as grease proof. It efficiently acts as barrier to moisture, germs, odors, and other contaminants. In addition, it acts as a total barrier to light and oxygen which are key elements in allowing fats to oxidize or become rancid, thereby successfully helping elongate the shelf life of food (“Foil and Packaging”). Alongside these aspects that help protect the quality of the product, the pliable and easy to manipulate nature of aluminum foil makes it an obvious choice as the ideal material to package the uniquely shaped Hershey’s Kiss.
Since the primary body of the packaging of a kiss consists of only one material, aluminium foil, at first glance it appears to be a very straightforward and simple packaging solution. It is easy to unknowingly overlook the number of raw materials and the sheer quantity of energy that goes into the cradle-to-gate cycle of making that single material. Aluminium foil is produced by rolling aluminium metal into sheets, however, despite accounting for 8.2% of the earth’s crust and being the most abundant metal in it, aluminium is never found free in nature but instead exists in compounds from which it has to be extracted (“The Element Aluminium”). The most significant aluminium ore is bauxite, which is a combination of aluminium hydroxide (a mixture of water and alumina) along with various mixtures of silica, iron oxide, titania, and other impurities. Though aluminium hydroxide is its primary component, it takes between 2 and 3 tonnes of bauxite to produce a single tonne of aluminium oxide (USGS). Since bauxite is found in abundance close to the earth’s surface, it is usually strip mined using explosives. Bauxite is classified according to its intended commercial application into categories including abrasive, cement, chemical, refractory, and metallurgical- the last of which is used in the production of aluminium foil (USGS). Two inventions in the 1880’s facilitated the widespread production and consumption of aluminium- the Bayer method of obtaining aluminium oxide from Bauxite and the Hall-Héroult method of obtaining aluminium from aluminium oxide (“Aluminium Life Cycle”). The bayer process utilizes sodium hydroxide, also known as caustic soda, to create sodium aluminate which is then crystallized into alumina. A range of other chemicals are also used during the process, depending on the concentration and nature of the impurities in the bauxite (“Aluminium Foil”). The next step in the production of aluminium is the Hall-Héroult method of smelting the alumina, thus obtained from the bayer process, in a carbon or graphite lined steel container called a “reduction pot”. This entails dissolving alumina in molten cryolite and electrolysing the molten solution using the aid of a carbon based cathode and anode. The positively charged anode is made of petroleum coke, while the carbon/graphite lined walls of the pot act as the negatively charged cathode. When electricity is passed through the solution, carbon from the anode combines with the oxygen from the alumina, leaving behind aluminium metal which can be cast into ingots, which in turn can be rolled into foil (“How Aluminium is Produced”). For the rolling process, a lubricant is required for the surface of the foil while it is being passed through the mill rolls. Kerosene is a common lubricant, however when the foil is being used for the intention of food packaging as it is in this case, the lubricant has to be a food safe and non toxic material such as a biobased lubricant or an oil-water emulsion (“Rolling Aluminium...”).
Along with the pyramidal shape enveloped in aluminium foil, the paper “plume” of each kiss is fundamental in making the product so easily recognizable. The thin strip of paper protruding from the foil helps facilitate the easy unwrapping of the chocolate as well as establishing brand identity by having the company name printed on it in order to distinguish a genuine kiss from look alikes. Since the paper comes in direct contact with the chocolate, it is essential for the material used for the plume to be a food safe as well as non-stick material. The paper used for the plume is extremely thin, has a non-stick surface, and a mild translucent quality. A variety of papers used in the food and photography industry embody these characteristics, including wax paper, butter paper,glassine, and parchment paper (also known as baking paper). An assumption made in order to further the investigation of the life cycle of a kiss is the use of parchment paper for the plume, as it is the most common grease proof paper used in the food industry and is closest in thickness and overall consistency to the paper “plume”.
Parchment paper is a cellulose-based paper similar in production and raw materials to ordinary paper, with the addition of certain materials to give it a non-stick quality. Different methods and materials can be used to create parchment paper, however the paper used in the food industry is primarily created as coated paper; the sheets of paper are produced from paper pulp and then coated with additional materials (Quandt and Newman). Pulp is considered to be one of the most abundant raw materials worldwide, and is a fibrous material obtained from two key ingredients- wood fiber and water. The wood fiber can come from a variety of sources; approximately half the fiber for production comes from pulpwood harvested directly from trees for the purpose of pulping, while the rest of the fiber comes from sawmills, recycled paper, recycled cloth, and plant/vegetable matter (Sixta). The sources of wood fiber are converted into small units, such as wood chips in the case of pulpwood, and then boiled in a solution of water mixed with chemicals and bleach.
In the case of parchment paper, the thin sheets of paper pulp are coated with a release agent. The most common release agent is silicone due to its low surface tension, which prevents things from adhering to its surface, as well as the fact that it is considered food safe (Walter, et al). Unlike silicon which is a naturally occurring chemical element, silicone is a synthetic substance (Castro). It is a polymer made up of the synthetic compound siloxane (which consists of a chain of alternating silicon and oxygen atoms) frequently combined with carbon and hydrogen (“Siloxane”). Silicon itself is made from the reaction of silica (silicone dioxide), obtained from the raw material metallurgical grade gravel, and carbon rich materials like coal, coke, or wood chips, in order for the silicon dioxide to reduce to silicon (“Silicon”). Thus the silicone coating of the parchment paper alone requires an extensive array of raw materials for its production, revealing the deeper impacts of the overall life-cycle of Hershey’s Kisses.
The plume of each kiss is printed with the trademarked name “KISSES” in blue ink. Though the text doesn’t always come in direct contact with the edible portion of the product, it is still essential for the entire plume to be food-safe and non toxic since it is in such close proximity (Barrientez and Strege). In addition, most food is organoleptically sensitive. Therefore the packaging of a food product should in no way influence the quality or taste of the product by imparting its own taste or smell. Food grade inks are a highly complicated and specialized part of any product packaging. A large proportion of food grade packaging is printed using solvent based ink systems, and for the purpose of this research project we will assume the same for this packaging. The composition of printing ink varies greatly based on a number of factors including the printing process, the demands of the finished product, the colors being used, the duration required, the environment the inks are exposed to, etc. However all inks consist of a basic formula consisting of a colorant, a vehicle, a solvent and assorted additive; for solvent based inks the solvent is usually ethanol or ethyl acetate, while the vehicle can be synthetic or modified natural resins such as nitrocellulose (Kunjappu). The colorants used in all inks are an assortment of pigments, which can be organic or inorganic. Since the KISSES logo printed on the plume is a uniform blue, a single pigment is used to produce the color. Blue ink can be obtained using the pigment copper phthalocyanine, which is a bright, crystalline, synthetic pigment commonly used in inks, paints, and dyes (Gregory).
Though the entirety of the packaging of a Hershey’s kiss is technically made up of only the three basic materials discussed in the research thus far - aluminium foil for the body, parchment paper for the plume, and blue ink for the text- once we examine the production of each of these materials from start to finish, the assumptions of the simplicity of its life cycle are dispelled. Even the most elementary products and materials we encounter on a day-to-day basis often have elaborate life cycles that make a much greater impact on our resources and environment than we are aware of.
Bibliography
A. D.McNaught and A.Wilkinson. "Siloxanes." IUPAC Gold Book . Blackwell Scientific Publications, 1997. Web. 12 Mar. 2016. <http://goldbook.iupac.org/S05671.html>.
"Aluminum Foil." How Aluminum Foil Is Made. Host website., n.d. Web. 01 Feb. 2016. <http://www.madehow.com/Volume-1/Aluminum-Foil.html>.
"Aluminum Life Cycle." The Life Cycle of Aluminum. Norsk Hydro, n.d. Web. 03 Feb. 2016. <http://www.hydro.com/en/About-aluminium/Aluminium-life-cycle/>.
Barrientez, Lisa, and Paul Strege. "Food Packaging Inks and Coatings: Achieving Safety and Compliance." Food Safety Magazine. Aug.-Sept. 2003. Web. 02 Feb. 2016. <http://www.foodsafetymagazine.com/magazine-archive1/augustseptember-2003/food-packaging-inks-and-coatings-achieving-safety-and-compliance/>.
Baughan, Joan and Keithline, Jeffrey “The Regulation of Printing Inks in the United States.” Keller and Heckman. Packaging Law. Web. 03 Feb. 2016.<http://www.packaginglaw.com/special-focus/regulation-printing-inks-united-states>.
Castro, Joseph. "Silicon or Silicone: What's the Difference?" LiveScience. TechMedia Network, 2013. Web. 13 Mar. 2016. <http://www.livescience.com/37598-silicon-or-silicone-chips-implants.html>.
"Foil & Packaging." The Aluminum Association. The. Web. 14 Mar. 2016. <http://www.aluminum.org/product-markets/foil-packaging>.
"How Aluminum Is Produced." Rocks and Minerals. 16 May 1999. Web. 12 Mar. 2016. <http://www.rocksandminerals.com/aluminum/process.htm>.
Kunjappu, Joy T. "Ink Chemistry." Chemistry World. March 2003. Royal Society of Chemistry. Web. 10 Mar. 2016. <http://www.rsc.org/chemistryworld/Issues/2003/March/inkchemistry.asp>.
Maria. "Hersheys Kisses Packaging." Message to the author. 08 Mar. 2016. Email from consumer representative at Hersheys.
Peter Gregory. “Industrial applications of phthalocyanines”. Journal of Porphyrins and Phthalocyanines. Vol 4 Issue 4. 2000. Word Scientific. Web. 07.March.2016.<http://www.worldscientific.com/worldscinet/jpp>.
Quandt, Abigail, and Newman,Walter. "Parchment Treatments" Paper Conservation Catalogue, 9th ed. Book and Paper Group, 31 May 94. Web. Cool Conservation. 08 Mar. 2016. <http://cool.conservation-us.org/coolaic/sg/bpg/pcc/18_parchment.pdf>.
"Rolling Aluminum: From the Mine Through the Mill." www.aluminium.org. The Aluminum Association, Dec.-Jan. 2007. Web. 10 Mar. 2016. <http://www.aluminum.org/sites/default/files/Rolling_Aluminum_From_The_Mine_Through_The_Mill.pdf>.
"Silicon." How Silicon Is Made. Host website, n.d. Web. 10 Mar. 2016. <http://www.madehow.com/Volume-6/Silicon.html>.
Sixta, Herbert. Handbook of Pulp. Weinheim: Wiley-VCH, 2006. Volume 1.
Thomas Jefferson National Accelerator Facility - Office of Science Education. "The Element Aluminum." It's Elemental . Web. 10 Mar. 2016. <http://education.jlab.org/itselemental/ele013.html>.
USGS. "Bauxite and AluminaStatistics and Information." Minerals Information: Bauxite. USGS. Web. 12 Mar. 2016. <http://minerals.usgs.gov/minerals/pubs/commodity/bauxite/>.
Walter,Henry et al. “Chap. 18 in Paper Conservation Catalog.” American Institute for Conservation Book and Paper Group. BPG, 31 May 1994. Web. 30 Jan. 2016. <http://cool.conservation-us.org/coolaic/sg/bpg/pcc/18_parchment.pdf>
Cynthia Chong
DES40A
Research Paper
March 14, 201
Embodied Energy used in the life cycle of Hershey’s Kisses Chocolate Packaging
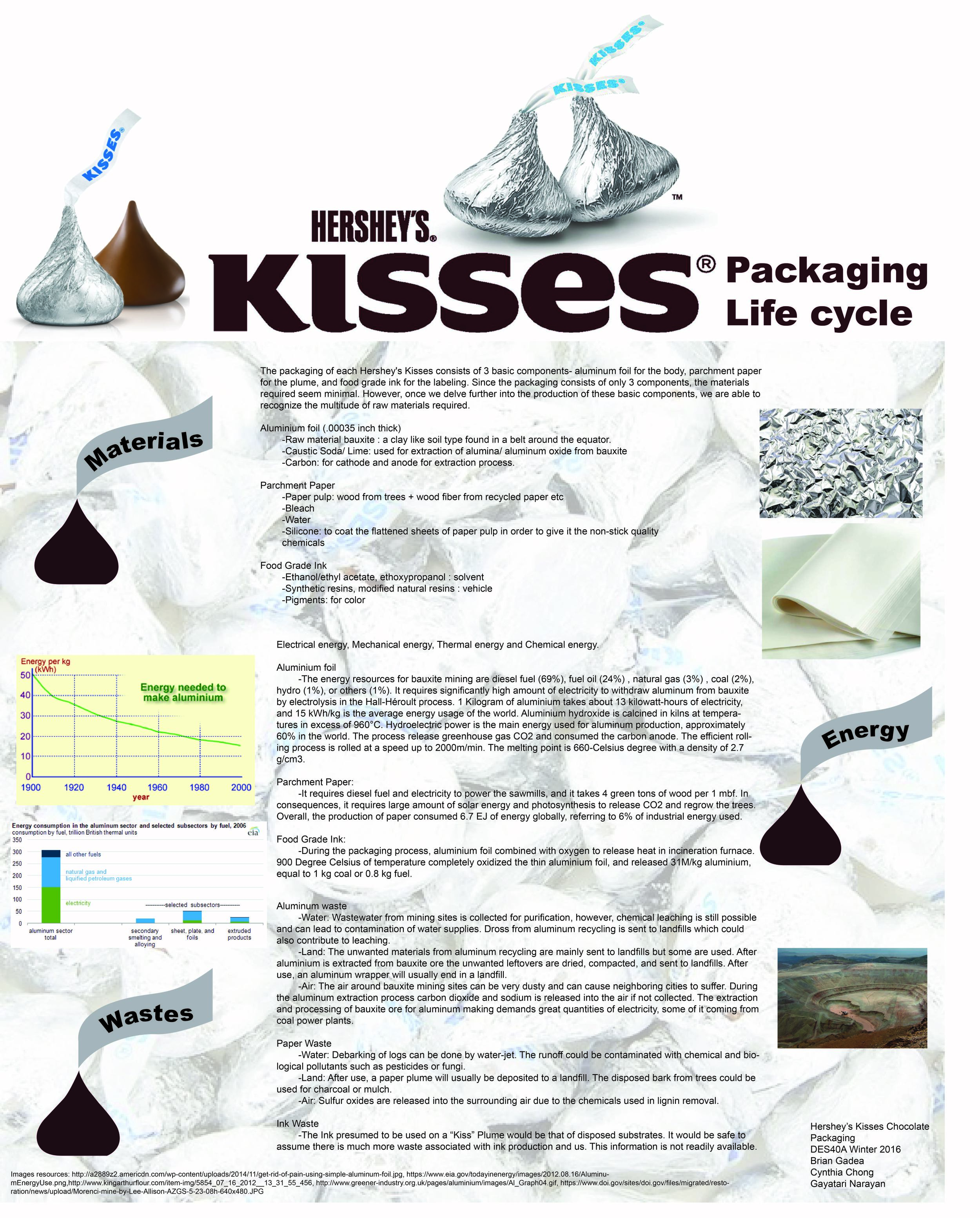
Hershey’s Kisses chocolate is wrapped by placing a strip of parchment paper with a word “kisses” printed on it in a 0.00035 inch gauge recyclable foil. The cone-shaped and the material design of the package successfully intrigued countless of consumers from all around the world. There are multiple forms of energy and different amounts of energy used during the productions of a wrapped Hershey’s Kisses chocolate. The energy used throughout each step of the life cycle of Hershey’s Kisses Chocolate Packaging includes the process of raw materials extraction, manufacturing and production, packaging and printing, maintenance and reuse, distribution and disassemble, and recycle and waste management.
The processes of raw material extraction used to create the aluminum foil and parchment paper of a Hershey’s Kisses Chocolate requires large amount of energy. Aluminum is the third most abundant metal in the earth’s crust, about 7%. Before the production of aluminum foil, aluminum has to be extracted from the raw material called “bauxite”. The bauxite ore contains 15-25% of aluminum in the form of aluminum hydroxide. To extract aluminum from bauxite involved intensive electrical energy, mechanical energy, solar energy and chemical energy. Therefore, bauxite mining is need for the extraction of aluminum. The energy resources for bauxite mining are diesel fuel (69%), fuel oil (24%), natural gas (3%), coal (2%), hydro (1%), or others (1%). However, aluminum cannot be extracted from bauxite directly through chemical processes, but rather through electrolysis in the Hall-Héroult process requiring a significantly high amount of electricity. The Hall-Héroult process is the major industrial machine for smelting aluminum, and it consumed a large amount of electricity because tons of amperes are used in each Hall-Heroult cells. 1 kilogram of aluminum takes about 13 kilowatt-hours of electricity, and 15 kWh/kg is the average energy usage of the world. On the other hand, the parchment paper is a sheet of paper pulp coat with silicone paper pulp. It is a sheet material made from the animal skin such as sheep and goats. Moreover, the fibers such as paper pulp needed for the production of paper are come from wood. In order to harvest the trees, it requires diesel fuel and electricity to power the sawmills, and it takes 4 green tons (8000lb) of wood per 1 mbf (1000 board-foot). As a result, the production of paper requires large amount of solar energy and photosynthesis to release CO2 and regrow the trees. After extracting the aluminum and paper pulp from the raw materials, it is able to start the manufacturing of aluminum foil.
The processes of producing aluminium foil include many steps, such as refining, smelting, rolling, and finishing. First, aluminum oxide (Al2O3) is refined from the bauxite by high temperature and is calcine in kilns at temperatures in excess of 960°C. The primary production process consumes about 14kwh per 1 kg of aluminum. Hydroelectric power is the main energy used for aluminum production, approximately 60% in the world, rather than non-renewable energy sources. As the above-mentioned, the Hall-Héroult process is the primary process for smelting aluminum. The process releases greenhouse gas CO2 and consumed the carbon anode. There will be a cold and heated container in the aluminum melting pot in 660-Celsius degree with a density of 2.7 g/cm3. The shape of the aluminum foil is created within an aluminum mold, and then scrolled. The efficient rolling process is rolled at a speed up to 2000m/min. In the process of rolling the aluminum foil, the aluminum is heated by rolling back and forth causing it to gets thinner and longer. The metal strip can be rolled 2 - 6 mm thick. Afterwards, the strip is cooled and rolled to 6 microns in thickness. It is the thinnest aluminum foil used for wrapping chocolates. Lastly, hot flue gases from the furnace are used in coating the rolled strip. The equipment involved in the entire manufacturing of aluminum foil includes rolling machine, doubler, separator, roll grinding machine, and annealing furnace. The rolling mill operation platform is the control center for all of the machines. As a result, the largest amount of energy used in the secondary production of aluminium foil is the thermal energy, which electricity conductivity up to 235 W/m K, and less energy intensive by comparing to the primary production.
The method of papermaking changed due to the times and advanced technology development. Originally, the production process of parchment was slow because of the long selection of good animal skin. Weak tanning solutions was applied to the skin surface then toughen the skin surface. Now, tanning agent is made mostly of the parchment paper from sheepskin. Physical and chemical stability of the parchment paper is very durable, which can bear the mechanical damages, such as the surface abrasion and tearing. In addition, the manufacturing process of papermaking include making pulp, beating, pulp to paper, and finishing. In the mechanical process of making pulp from converting logs to wood pulp, bark is first removed from logs; the logs are then sent to grinders. Then, by pressing it between huge revolving slabs, the logs break the wood down into pulp. On the other hand, in the chemical process, the wood chips were boiled at high pressure in a solution of sodium hydroxide and sodium sulfide. The pulp is sent to the paper plant after the pulp is filter and adds the bleach. In the process of beating, the chemicals such as titanium oxide can be added, and it relatedly influences the opacity and other qualities of the paper. Most important, sizing will affects the way the paper will react with different kinds of inks. In the process of manufacturing from pulp to paper, pulp is fed into the machine called “Fourdrinier”, then squeezed and pressed through chain of rollers of wood felt. Then paper then may pass over a series, steam-heated cylinders to remove all of the water. Finally, in the process of finishing, the dried paper is smoothening by passing through metal rollers called calendars. Coating will be added and the paper is ready to cut in the desired size. In overall, the manufacturing of producing paper and pulp has a significant high electricity energy used and emissions of CO2, which the estimated Combined Heat and Power (CHP) is 0.3-0.6 EJ/year.
In order to print the word “kisses” onto the paper, it requires an operation of inkjet printing. Liquid ink in blue being sprays on paper creates the brand name. It takes half a second to print the strips. Moreover, “Drop on demand” (DOD) is the most common type of inkjet printing being used in manufacturing, and squirts small droplets of ink onto paper through tiny nozzles. The printing process uses thermal technology, which heat is used to fire ink onto the paper. Primarily, the ink is heated to create a bubble, and then it bursts under the pressure force, and hits the paper to initiate the squirt. The bubble then collapses as the element cools, and the vacuum draw inks to replace the ink that was ejected. The heating elements are used to eject ink droplets from the print head’s nozzles, and most thermal inkjets have print heads containing 300-600 nozzles. No matter what kind of ink is used, the printing process required thermal energy because it must be heat-resistant and the firing process is heat-based. Therefore, thermal printers require a cooling process and it add a length to the overall printing time. For the color print, fast-drying ink is more preferable as they are 100 times faster than the slow-drying ink in order to avoid the blurring of words. The inkjet printing used water-based ink instead of oil-based ink as it reduce higher maintenance cost. Overall, the production of paper consumed 6.7 EJ of energy globally, referring to 6% of industrial energy use.
Hershey's Kisses chocolates wrapped in aluminium foil is the lightest packages that combined with paper and plastic laminates. There is estimation that 80,000,000 Hershey's Kisses chocolates are wrapped each day, the amount of aluminium foil used each day can cover up to over 40 football fields. Hershey's Kisses chocolates were originally wrapped by hand. This process of hand-wrapping a kisses chocolate is required to lay a piece of tissue paper on a sheet of foil, then place the chocolate on and twist the wrapper. However, this method was too slow and difficult to paid worker’s salaries by the quantities of wrapping each chocolate. The wrapping machine was developed in 1921, and the machines can wrap to 1300 kisses chocolate per minute. The aluminum alloy sheet has a thickness between 0.004 and 0.24mm. Besides, the paper strips call “plume” is automatically insert in the wrapping process by machines because it allows consumers easier to open the foil and recognize it is Hershey's kisses chocolate. In general, these machines used of mechanical energy and electronic control system. The paper stocking in these machines has to be very thin and the machines are sensitive to the humidity, ink coverage and thickness of the wrapper. Most of the machines have a top wrapping speed in the 100-200-bars/minute ranges, depending on bar size. To wrap a huge amount of Hershey's Kisses chocolates, it requires tons of wrapping machines and operators for changing foil and paper rolls. In specific, Hershey's Kisses chocolates used of horizontal flow-wrapping machines in the wrapping process. It formed by fusing the aluminum foil with heat and pressure by squishing together surfaces with pre-applied cold contact adhesive. Because Hershey's Kisses chocolates produce various kinds of chocolates, they design each packaging line capable of packaging different products, and it have to be flexible and reliable, so that the products can be easily adjusted and reduce the chances of waste packaging. The main three elements for the packaging process are heat, dwell, and pressure. With the advanced heat and seal technology, it have better temperature control and it reduce the time consuming for Hershey's Kisses chocolates packaging.
Besides the classical silver foil color, different varieties of coloring foil wrappers began in 1962 since the color revolution. The packaging colors are introduced by seasons or holidays, such as red and green are manufacture for Christmas season while wrappers of pastel blue, pink and green are manufacture for Easter. The packaging printing used of solvent based ink systems with food grade ink. Beyond the materials, the printing techniques of papers and aluminum foil are the same, which use the same types of plates and the same presses. The printing process is called rotogravure. Rotogravure printing on aluminum foil can achieve a high quality of colors and uniformity with the widest range of colored transparent inks. It is easy to process and laminate. Beside, it is an economical process for mass production of printing. By comparison, ink drying on foil is slower than on paper. The optional energy used for drying system is thermal oil, gas burner, and electric heater. During the packaging process, aluminum foil combined with oxygen to release heat in in incineration furnace. 900 degree Celsius of temperature completely oxidized the thin aluminum foil, and released 31M/kg aluminum, equal to 1 kg coal or 0.8 kg fuel.
Aluminum foil packaging for Hershey's Kisses chocolates is non-toxic and durable, which will not affect the taste of the chocolate but as a protection. Also, the chocolate can maintain for 2 years or more if wrapped by the foil. Aluminum foil does not melt from high temperature, however, the chocolate that wrapped inside will melt and the shape will be damage if it encounters thermal energy. In contrast, if keep the kisses chocolate are put in the refrigerator, the aluminum foil will have a long-lasting shield life. Moreover, the parchment paper can withstand a dry heat of 100 degree Celsius without changing the color and the dimension of the shape in thermal reactivity. However, moist heat can lead the parchment paper to swell, decreasing the age of the paper and related to shrinkage temperature.
Apart from the aluminum foil is a suitable packaging materials, it is also a convenient materials for distribution and transportation because it is lightweight. Aluminum foil is a lightweight material that used fewer energy and fuels for the manufacturing process compare to other primary metal produced from bauxite. Shipment of aluminum foil began in 1991. In terms of each Hershey's kisses chocolate packaging, if they need to be distributed in far locations, they would probably shipped by plane. Within local areas, it will distribute by truck. After customer purchases the kisses chocolate in the store, they will drive them home by car. Therefore, the largest energy resource used for the transportation is fuels and kinetic energy.
Last but not least, aluminum foil is an easy-to-recycle and a reusable material. Aluminum packaging consumed less than 10 % environmental impact in Hershey's Kisses chocolates life cycle. Recycling the recovered aluminum can reduce the heavy reliance on natural resources. Thin aluminum will be recovered through incineration, minimize the amount of waste sent to landfill. During the incineration process, in particular the thin and laminated foil fraction is oxidized, and converted energy to heat and electricity. To maintain the purity, the recycled aluminum is combined with pure aluminum. In consequences, recycling aluminum can reduce money on energy consumption, such as the extraction of raw materials, and keep the shapes of landfill by less mining. Compared to the manufacturing of new aluminum, the recycling can saves a lot of heat and electricity energy, as well as CO2. Therefore, the reuse of aluminum provides higher energy efficiency. Since 1980, the recycling industry played an important role in aluminum life cycle. The aluminum recycling industry included refiners, produce alloys from customer’s specifications, and smelters, produce from the same type of materials. The recycling industry are also include collectors, dismantlers, metal merchants, and scraps processors. Even though the aluminum foil of Hershey's Kisses chocolates is thin and has low aluminum content, they still can be extracted from laminates by special techniques. One tons of recycled metal is equal to 14,000-kilowatt hours of electricity, 2,663 gallons of oil, and 10 cubic yards of landfill space. Practically, different mechanical, thermal, chemical, and magnetic energy are used in the aluminum recycling process.
In conclusion, there are different kinds of energy resources and huge amounts of energy being used in each process of the production of Hershey’s Kisses Chocolate Packaging throughout the entire life cycle. Even though Hershey’s Kisses Chocolate seems to be a simple product, it takes a lot of energy and resources from the earth that beyond our imagination. Hopefully, the efficiency of the production can increase relatively with the high usage of energy while time consuming of the manufacturing can decrease through the advanced development of technology.
Bibliography
“Hershey's Kisses.” Wikipedia. Wikimedia Foundation, Inc. 7 Jan. 2016. Web. 1 Feb. 2016.
“1921Machine Wrapping.” Our Story. Hershey’s Kisses, December 05. 2014. Web. 1 Feb. 2016.
“Printing/Coloring on Aluminum Foil.” Converting Materials and Methods. www.aluminumfoils.com, 2005-2013. Web. 1 Feb. 2016.
“Hershey`s marketing plan report.” LinkedIn SlideShare. LinkedIn Corporation, Nov 09. 2013. Web. 1 Feb. 2016.
“Ariflex - Aluminum Foils for Flexible Food Packaging.” packaging-gateway.com. 2016 Kable, a trading division of Kable Intelligence Limited. Web. 1 Feb. 2016.
“Aluminum life cycle”. About Aluminum. Norsk Hydro ASA, January 21. 2013. Web. 2 Feb. 2016.
“Foil & Packaging.” Product Markets. The Aluminum Association. Web. 2 Feb. 2016.
Mohan, Samarth. “How are Hershey's Kisses wrapped?.” Quora. Quora, Sep 19. 2014. Web. 2 Feb. 2016.
Speer, Matthew. “Facts About Aluminum Foil Reuse and Recycling.”Sustainable Living & Sustainable Lifestyle. iFame Media, LLC, Feb. 24. 2012. Web. 2 Feb. 2016.
“Foil for technical applications.” Rolled Products. Norsk Hydro ASA. Web. 2 Feb. 2016.
“Electricity consumption in the production of aluminium.” MrReid.org, 15 July. 2015. Web. 10 Mar .2016
Kinney, Suz-Anne. “Dispelling the Whole Tree Myth: How a Harvested Tree Is Used.” F2M Market Watch. Forest2Market, Inc, 20 Dec. 2013. Web. 10 Mar. 2016.
“Aluminum - Aluminum Foil Production.” AZoM materials. AZoNetwork UK Ltd., 11 Jun. 2013. Web. 10 Mar 2016.
Noble, Steve “Digital Printing.” Printers’ National Enviormental Assistance Center, PNEAC, 1 Jul. 2011. Web. 10 Mar 2016.
“Pulp and Paper.” Industrial Efficiency Technology Database, The Institute for Industrial Productivity, 17 Feb. 2011. Web. 10 Mar 2016.
Whitaker, Shane “Horizontal Wrappers Seal for Perfection.” Operations. Sosland Publishing co., 1 Mar. 2012. Web. 10 Mar 2016.
“ALUMINUM IS GREEN.” Western Plastics, Web. 10 Mar 2016.
Akhmadulina, Bella “ALUMINIUM IN PACKAGING.”All about aluminum, UC RUSAL. Web. 10 Mar 2016.
Robertso, Gordon L. Food Packaging: Principles and Practice. CRC Press, 2012. Print.
Brian Gadea
The Waste of a Hershey’s Kiss
Throughout this paper I will record data yielded through research regarding the wastes or “byproducts” of Hershey’s Kisses’s aluminium foil wrapper and it’s paper “plume.” I will organize the details in “Chronological” order in regards to that of the production process for each of the following packaging item; Aluminium foil, glassine paper, and printing ink. The Hershey’s company partners with multiple companies as suppliers. I can only infer that their business partners change over time as a complete list of suppliers is not readily available. Thusly, the transportation methods these companies use will be estimated. I believe that what becomes waste could be a viable resource. Learning what these byproducts are is a step towards increasing efficiency. It is my personal belief that we are also responsible for cleaning up after ourselves as best as we can.
I will begin with the mining process. It begins with land surveying. In order for a wrapper of any kind to be made the raw materials to make them must be abundantly available. To be sure that any raw material needed is available a supplier will have to conduct tests in order to choose a viable source or sources. In the case of Hershey’s Kisses single wrappers the main sources would be: bauxite deposits and forest areas (the bauxite is used for foil and the forests are used for paper). According to web.mit.edu a mining company would have two methods of surveying: feasability study or Greenfield exploration.
In feasibility studying either a small team of individuals or a single person who is/are qualified is/are sent to an area of land already known to be a source of bauxite ore (web.mit.edu). The person(s) are to obtain drill samples, survey for ground stability, research cost of mining permits, research cost of re-forestation, etc.
In the case of Greenfield exploration there are tests done in order to first find ore deposits through the use of radiation detection, magnetism measurements, gravimeter measurements, satellite images, and rock sampling (web.mit.edu). Both of these methods entail the use of fossil fueled energy and with it its emissions; whether it be exhaust from machinery, exhaust from power generators, and even airplane and truck exhaust. These methods also produce some quantities of sound pollution depending on the machine(s) used. There can also be possibility of water contamination from sample drilling.
Once a profitable land area is agreed upon mining can commence. The mining area is cleared out of any unwanted vegetation, animals, and unwanted topsoil(s) (aluminium.org.au). During this process the machines used will emit fossil fuel exhaust from diesel, propane, and other natural gases. Diggers, generators, drills, belts, tractors, trucks, cranes, and other heavy lifting machinery is used every day until project completion. The clear cutting sometimes gives opportunity for the plant matter to be sold or used on-site otherwise the remnants of the vegetation and animals are left aside.
After the land is cleared of vegetation and animals the extraction of topsoil commences and gives way for three other possible “side effects:” sound pollution, water pollution, and dust. The mining area is now frequent with activity and with it comes high noise pollution. Continuous gears, engines, drills, explosions etc. all cause high noise levels. As noise is not channeled for production I will consider it a “waste product.” Some companies, such as Alcoa, state that they do attempt to alleviate the sound issue by reducing the decibels that each part of the process produces and by limiting their work hours. Many mines also use water to clean or to help dissolve and cut through dirt or rock. Varying types of water contamination such as the leaching of dangerous chemicals or mineral runoff into any local body of water or water supply are always a risk. Further on, the clearing of vegetation along with the combination of wind and vehicle movement all contribute to dust in the air. In order to help alleviate the dust buildup water is sprayed on the ground to keep it from dusting. Although this method helps it does not eliminate the dust completely. After the topsoil is cleared the bauxite extraction commences. The ore is now delivered to a processing plant.
Once the bauxite ore is delivered to a processing plant the aluminium is to be extracted. The bauxite is introduced into a grinding mill. The finely ground bauxite is then sent to be “digested.” The bauxite is poured into a caustic soda solution which dissolves the aluminium oxide within the ore. The dissolved material makes its way into the next tank as a solution. The solution now sits in “thickening tanks” to allow for any impurities to settle at the bottom and allow for the purer substance to pass through. This residue accounts for the largest solid waste of the extraction process (primary.world-aluminium.org). “It is primarily composed of the insoluble fraction of the bauxite ore that remains after extraction of the aluminium... Iron oxides (10 – 30%), titanium dioxide (2– 15%), silicon oxide (5 – 20%) and undissolved alumina (0 – 20%) make up the residue…” (primary.world-aluminium.org). The settled impurities thicken, the thick material is extracted, and separated from the alumina solution, then it is dumped into a disposal area or treated to become “well structured” soil (bauxite.world-aluminium.org). The solution is filtered several times then is transferred to tanks where alumina crystals can form. At the same time the caustic soda is extracted and may be recycled and re-used afterward. The crystals are extracted then heated to over 1000 ℃ using gas to produce purer alumina crystals as powder.
Now that the alumina has been extracted it can be converted into aluminium. This part of the process requires mass amounts of electricity and thus has a high demand for power. To begin, alumina, molten cryolite, and other materials are poured into a rectangular carbon block (Aluminium.org.au). Now an anode is used to conduct electricity through the molten mixture. Throughout this process carbon dioxide, sodium, and cryolite particles are released but are usually collected and kept from being released into the atmosphere. In some cases, however, the materials are released into the air of the factory (Aluminium.org.au). The aluminium now separates and is “tapped” from the carbon container and transported to be cast into molds. It is then prepared for shipping to a foil making factory.
Aside from virgin aluminium, recycled aluminium might also be used in foil making. The recycling process does emit certain waste. The aluminium recycling process creates dross. Dross is a mixture of chemicals that may include: SiO2, CaO, Na2O, Al2O3, Fe2O3, and many others (Adeosun et al. 7). These are separated from aluminium during the recycling process. The chemicals left behind come from alloys, paints, plastics, basically anything that has been added to the aluminium by product design. Currently the dross itself can be used. It has been added to concrete to add strength, for example (Dai 34). The use of dross is currently experimental and does have potential applications. Although there are uses for dross, much of it is still sent to landfills which can contribute to land and possibly groundwater pollution. For further information on the subject I recommend reading Dai’s thesis on dross. Further on, aluminium recycling also contributes to the burning of fossil fuels, possible noise pollution, and air pollution from the burning process.
The ingots are transported to a foil making factory by plane, truck, or boat. Once again, fossil fuels are used for transportation and thus the emissions of the boats and planes used for transport are also part of what I would consider as a “waste” of this whole process. Once the ingots arrive at the factory they can now be melted, reformed, and pressed to form aluminium foil. The ingots are melted into a large block of aluminium using natural gas. The block is then sent through a long line of rollers until it is pressed to the width of aluminium foil. The foil is packaged and prepared for distribution to Hershey’s factories.
If the foil wrapping is disposed of into a recycling receptacle after use it might not be remelted. It could, theoretically, be melted down and re-used on any aluminium based product. Unfortunately, the recycling of aluminium foil can be difficult because it is cumbersome to extract only the aluminum from the product. When it comes to candy wrapping, any food particles, resins, paper, etc. must be separated from the metal, herein lies the problem. Many recycling plants are not equipped to separate every single particle from foil. This separation challenge is being addressed at this time and there have been proposed solutions. The technologies are still in development. It is much more likely that the wrappers will be sent to a landfill.
All of these processes require tremendous amounts of electricity. To function they mainly rely on a combination of hydroelectric and coal power (world-aluminium.org). Some factories also house wood powered furnaces or use wood as an energy source. The ash produced from these sources is sometimes sold as ground fertilizer
Our next focus will be that paper used in the “plume” on the Hershey’s Kisses. There are various methods for general paper making as each paper company has their processing preferences. I will attempt to cover the most appropriate method and reference any other methods. The use of recycled paper is a possibility but unlikely in this situation so it will be excluded. I will assume that Hershey’s has more than one supplier of paper products and that some would use recycled materials. A complete list of Hershey’s suppliers is not available to me.
Logging or paper companies prefer coniferous trees as their fibers are better suited for making paper and choose cutting sites appropriately. Once cut down, the logs are loaded onto trucks for distributing to paper processing plants. After arrival the harvested logs must be debarked (bark removal). They can be debarked through the use of water jets or rotating drums. This machinery does produce loud noise if not housed indoors and as it is also not channeled towards production I consider it a “waste product.” It should be noted that there may also be “infected” bark (that with mold, mites, etc.) that is removed. Any water “contaminated” through water jet runoff is to be disposed or treated properly. The bark that has been removed can now be sent to a landfill or used to provide charcoal for factory use (or sale), mulch, or ground cover. If used for factory power, then the ashes produced will be sent to a landfill or used for ground fertilizer, on farms, for example. This method of power will also expel carbon dioxide, heat, water vapor, and a few other chemicals. Now the debarked logs can be ground, by saw, to create wood shavings. This process can also produce long periods of heavy noise pollution, especially if not housed indoors. The wood shavings are then mixed and heated in various dissolving chemicals used to separate the wood’s fibers through lignin removal. It is during this stage that any recycled paper pulp might be added. Here we have chemicals that, after use, create unusable residuals that are highly corrosive in the form of sulphur oxides (ilocis.org). These residuals are then released into the air. Such airborne byproducts are known to cause damage to plant life within the surrounding area of a factory.
Paper factories also produce other unwanted chemicals such as: nitrogen oxide, nitrogen oxides, carbon dioxide, sulphur oxides, sodium sulphate, sodium carbonate, and other particulates (ilocis.org). Other gaseous compounds can also be minor contributors to mill air pollution: carbon monoxide from incomplete combustion, chloroform from bleaching, and volatile organics due to digester and liquor evaporation (ilocis.org). Finally, in order to get the type of paper that the paper plume is made of, a line of rollers must be used. As you might expect the rollers require electricity to function.
Lastly, there is the ink on the paper plume. The ink’s specific components are unknown nor is the method of printing available. I have only been told by a company representative that the ink is food grade ink. I will assume that Hershey’s uses ink jet printing and will describe general food ink based wastes. Food grade ink wastes are also not detailed explicitly. I could only find enough information on the subject to infer its waste. Ink waste would most likely come from substrates, working without volatile organic compounds, and without using pre-coats (De Mondt).
After all the materials are ready, Hershey’s then uses the foil along with their paper “plume” to machine wrap their Hershey’s Kisses. The Kisses are now bundled and packaged into bags of plastic ready to be distributed by truck, by train, or by air.
As technology advances these particulates, the contaminated waters, the harmful practices in general are reduced but not completely abolished. It is my belief that we are responsible for continuing as a species with a less intrusive model. We must find much more efficient ways of production and attempt to restrain our push forward as to not endanger the wellbeing of all that reside on our planet.
Further Reading and Information
http://www.hydro.com/en/About-aluminium/Aluminium-life-cycle/Bauxite-mining/
http://www.alcoa.com/australia/en/pdf/Noise_Management_Fact_Sheet.pdf
http://web.mit.edu/12.000/www/m2016/finalwebsite/solutions/deposits.html
https://www.youtube.com/watch?v=f4OTj9yNOak
https://www.youtube.com/watch?v=E4C3X26dxbM
http://www.madehow.com/Volume-2/Paper.html
http://homepages.wmich.edu/~maltby/PAPR3531/lectures/wwtp-residuals.pdf
https://www.youtube.com/watch?v=YiVFVRUFY24
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2538142/?page=3
Works Cited
Adeosun, Samson Oluropo, Olatunde Israel Sekunowo, Omotayo Oluwaseyi Taiwo, Wasiu Ajibola Ayoola, and Adebowale Machado, eds. "Physical and Mechanical Properties of Aluminum Dross." Advances in Materials 3.2 (2014): 6-10. Article.sciencepublishinggroup.com. Science Publishing Group, 10 July 2014. Web. 5 Mar. 2016. <http://article.sciencepublishinggroup.com/pdf/10.11648.j.am.20140302.11.pdf>.
"Alcoa in Australia: About Alcoa: Bauxite Mining: Environmental Management: Noise Management." Alcoa in Australia: About Alcoa: Bauxite Mining: Environmental Management: Noise Management. Alcoa, n.d. Web. 04 Mar. 2016. <http://www.alcoa.com/australia/en/info_page/mining_noise_management.asp>.
"Aluminium Flowchart." Alumina Refining. Aluminium.org.au, n.d. Web. 04 Mar. 2016. <http://aluminium.org.au/flowchart/alumina-refining.html>.
"Aluminium Flowchart." Aluminium Smelting. Aluminium.org.au, n.d. Web. 02 Mar. 2016. <http://aluminium.org.au/flowchart/aluminium-smelting.html#>.
"Aluminium Flowchart." Bauxite Mining. Aluminium.org.au, n.d. Web. 02 Mar. 2016. <http://aluminium.org.au/flowchart/bauxite-mining.html>.
"Aluminium Flowchart." Recycling Aluminium. Aluminium.org.au, n.d. Web. 04 Mar. 2016. <http://aluminium.org.au/flowchart/recycling.html>.
"Aluminium Flowchart." SemiFab and Products. Aluminium.org.au, n.d. Web. 03 Mar. 2016. <http://aluminium.org.au/flowchart/semifab-products.html#>.
"Bauxite Residue Management." Mining and Refining – Bauxite Residue Management. The International Aluminium Institute, n.d. Web. 27 Feb. 2016. <http://bauxite.world-aluminium.org/refining/bauxite-residue-management.html>.
Dai, Chen. "Development Of Aluminium Dross-Based Material For Engineering Applications." Thesis. Worcester Polytechnic Institute, 2012.Development Of Aluminium Dross-Based Material For Engineering Applications. M.wpi.edu. Web. 4 Mar. 2016. <http://m.wpi.edu/Pubs/ETD/Available/etd-010612-155135/unrestricted/cdai.pdf>.
"Emissions and Wastes." Aluminium for Future Generations – Emissions & Waste. The International Aluminium Institute, n.d. Web. 04 Mar. 2016. <http://primary.world-aluminium.org/aluminium-facts/emissions-waste.html>.
Fenrick, Greg. "Drilling." Drilling and Equipment Technology in Mining. TechnoMine, n.d. Web. 27 Feb. 2016. <http://technology.infomine.com/reviews/Drilling/welcome.asp?view=full>.
"Is Current Mining Yield Sustainable?" Mining. Web.mit.edu, n.d. Web. 29 Feb. 2016. <http://web.mit.edu/12.000/www/m2016/finalwebsite/solutions/mining.html>.
Kumar, Amit. "Feasibility Studies." Feasibility Studies in Mining. TechnoMine, Mar. 2012. Web. 23 Feb. 2016. <http://technology.infomine.com/reviews/FeasibilityStudies/welcome.asp?view=full>.
Mondt, Roel De. Digital printing: Low Migration inkjet inks for indirect food packaging Presentation. Digital image. Http://www.ilsi.org. Agfa, Nov. 2012. Web. 9 Mar. 2016. <http://www.ilsi.org/Europe/Documents/De%20Mondt.pdf>.
"PRIMARY ALUMINIUM SMELTING POWER CONSUMPTION." Http://www.world-aluminium.org. World Aluminium, 30 Sept. 2015. Web. 4 Mar. 2016. <http://www.world-aluminium.org/statistics/primary-aluminium-smelting-power-consumption/#map>.
"Using Environmentally Conscious Mining Standards." Green Mining. Web.mit.edu, n.d. Web. 29 Feb. 2016. <http://web.mit.edu/12.000/www/m2016/finalwebsite/solutions/greenmining.html>.
"Waste Management in the Pulp and Paper Industry." Advances in Hazardous Industrial Waste Treatment. Ed. Lawrence K. Wang, Nazih K. Shammas, and Yung-Tse Hung. Boca Raton, FL: CRC, 2009. 462+. Print.
"What Are the Water Quality Concerns at Mines?" Water Quality in Mining – MiningFacts.org. Miningfacts.org, n.d. Web. 04 Mar. 2016. <http://www.miningfacts.org/Environment/What-are-the-water-quality-concerns-at-mines-/>.