Design Life-Cycle
assess.design.(don't)consume
Joseph Goodwin
Professor Christina Cogdell
Design 40A
1 December 2016
Life Cycle Analysis: Materials of a Basketball
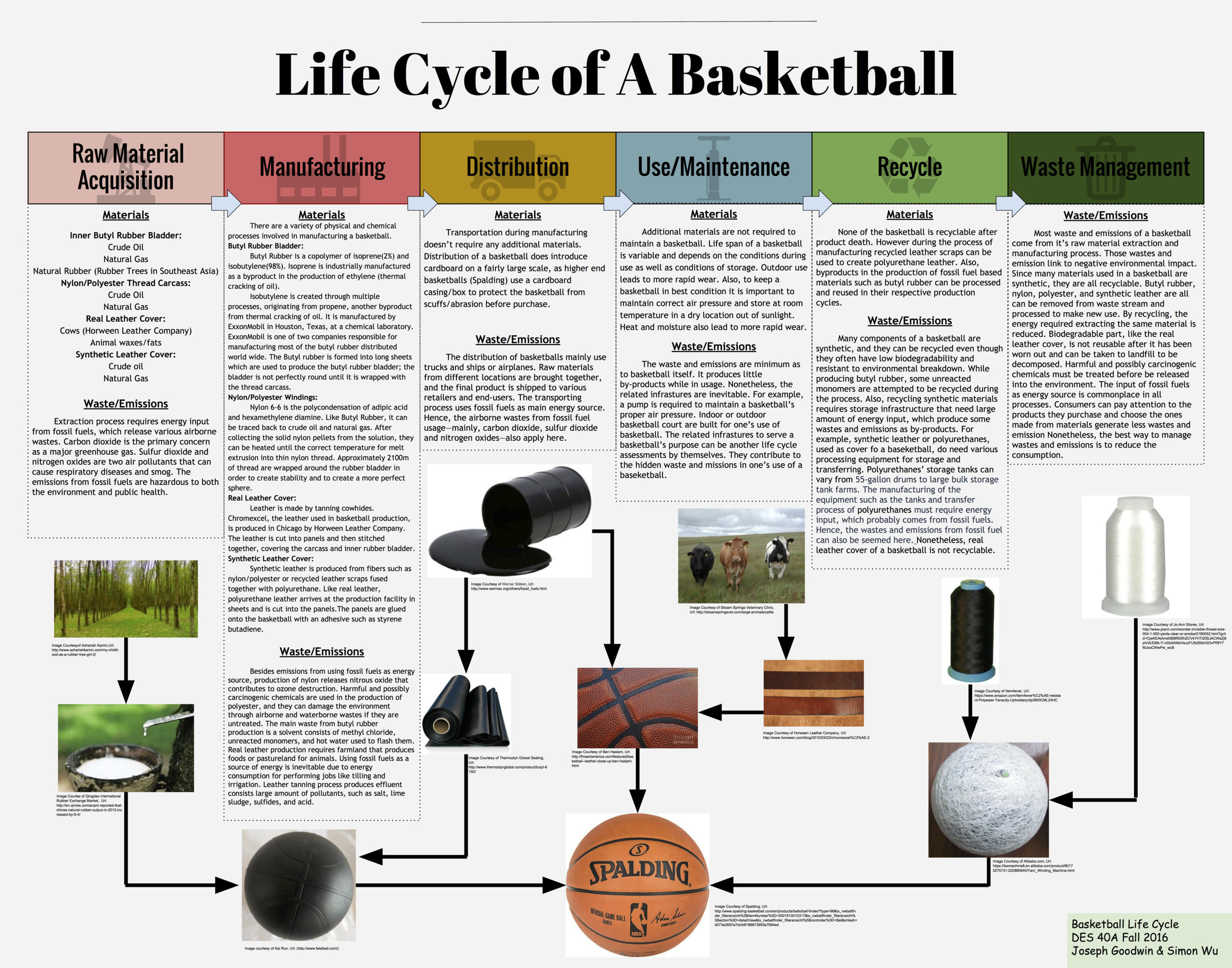
Over the past few years basketball has begun to rapidly grow in popularity. It's a fast paced game that can be exhilarating to watch, but few people consider the materials used to create the basis of the sport; the basketball. The modern basketball has 3 main components; a rubber bladder, a leather/composite cover, and a nylon/polyester carcass. Recreational balls have a synthetic leather or polyurethane leather cover while professional ones are made with real leather. The material chosen does change the properties the ball exhibits during use. For example a leather ball will bounce higher than a synthetic leather one, but the synthetic leather one is far less slippery. For manufacturing purposes, synthetic leather basketballs have replaced leather in order to keep costs low and profits high. One brand of basketball I will be referencing is Spalding, as they are the brand that produces the ball for professional play (NBA) as well as many different recreational versions. I will first examine each component of a basketball separately to isolate the raw materials acquisition as well as the manufacturing/processing required to get to the production of a basketball, as this is where a majority of the materials aspects of a basketball’s lifecycle is focused. I will then touch on both the use of a basketball as well as the end of its life cycle. A simple product like a basketball may not contain many different material components, but further examination of its lifecycle reveals the extensive story behind the materials that create an iconic product.
The rubber bladder is by far the most consistently manufactured part of a basketball in terms of materials used. Almost all basketballs have a butyl rubber/natural rubber bladder. Butyl rubber is ideal because of its high impermeability to air which allows it to hold air without losing any considerable pressure. Another quality that is essential is its elasticity, which is responsible for the ability to bounce off the ground to an height useful for play. Natural rubber has an even greater elasticity and allows for a bounce that loses even less energy and returns to an even higher height. Over time it has become standard to use a mixture that is predominately butyl rubber with some natural rubber in order to maximize the air retention as well as the rebound height. A ratio of 85% butyl rubber to 15% natural rubber is considered standard. The inner bladder is responsible for a large portion of the basketball’s overall weight. To put this in context, the inner bladder usually weighs somewhere between 140-150g while the total weight of a deflated basketball is somewhere between 465-475g. This means that butyl rubber is about a quarter of the total weight.
The primary materials of butyl rubber are crude oil as well as natural gas, which are found and extracted in various locations around the world. It takes several chemical processes as well as multiple additives to reach the final product. Because of this, it was very difficult to attempt and pinpoint an exact location in which the primary materials were extracted. Butyl rubber is a copolymer composed of 98% isobutylene and 2% isoprene. Isobutylene is produced through several processes, originating from propene collected from thermal cracking of crude oil. Similarly, isoprene is a byproduct of ethylene that is produced during thermal cracking as well. Exxon Mobile and Polysar Rubber Corp are responsible for producing most of the butyl rubber for the world. The Exxonmobil chemical production laboratory is based in Houston, Texas.
Conversely to the obscurity in the original material extraction location of crude oil, 90% of the world’s natural rubber is produced in Southeast Asia (“Rubber Faqs”). Natural rubber can be extracted from a variety of different plants, but it is most abundant in the rubber tree which favors humid equatorial locations. In fact, 99% of natural rubber is produced from the rubber tree (Woodford, Chris). After extraction from the tree, the rubber is a liquid-sap that needs to be filtered and reacted with an acid in order to create the solid rubber we are familiar with. From here, the butyl rubber from Exxonmobil and the natural rubber from Southeast Asia must be transported to QingDao, China in order to produce the inner bladder of the basketball (Bindy).
Most basketball covers are typically made from leather or composite leather such a polyurethane leather. The material in choice is determined usually by the environment the ball will be used in, as well as whether it will be use for professional/recreational use. Synthetic polyurethane leather has taken over as the predominant choice for production, in order to keep the prices low as well as profits high. This doesn’t mean that leather cover basketballs have become obsolete however, as the National Basketball Association (NBA) still uses real leather basketballs. In 2007 they tried to implement polyurethane leather basketballs, but the slight difference in rebound height was enough to bother the players, so they quickly reverted back to real leather.
Higher end basketballs are manufactured using real leather. For these top tier basketballs, leather represents the only other material that is not synthetic. The leather used for these basketballs can be traced back to the United States. High quality basketballs manufactured for the NBA are made using Chromexcel, a leather often used in sports. The leather is produced in Chicago by Horween Leather Company. The company is also responsible for producing the leather used in manufacturing NFL footballs. One thing to note about production of basketballs using real leather is the very small amount of leather scrap waste; this is due to the high cost of the Chromexcel leather. Maximizing their profits has lead companies to cut the panels of a basketball in ways that leave little to no leather scraps.
Recreational basketballs are created with synthetic leather covers such a polyurethane leather. Polyurethane leather and other alternatives provide additional durability for play outside, as well as weather/sunlight resistance that leather simply does not exhibit. On top of this, it is much cheaper for the companies to produce this, adding to the paradigm shift from real leather to synthetic leather over the past half century. Polyurethane leather is made from a base of fibers such as leather scraps or nylon/polyester all sealed together with polyurethane. There is an additional thin polyurethane layer on the surface. The polyurethane creates a surface that mimics the moisture absorbing effects of a real leather basketball. Unless the polyurethane leather is made from leather scraps, this portion of the life cycle begins from crude oil and natural gas much like the other components of a basketball.
A nylon/polyester carcass is positioned in between the bladder and the cover of the basketball in order to help maintain the shape of the ball and add needed durability. Without it the butyl rubber would have a high tendency to deform over time, creating lumps on the basketball that ruin the functionality of the ball. The windings help to prevent these deformations and keep the basketball perfectly round, allowing for smooth bounces on all sides. Although the windings add durability, they do reduce the overall rebound height of the basketball. A typical carcass is made from winding thread made from 60% Nylon and 40% polyester around the inner bladder. A complete carcass has about 2100m of 1 micron diameter wound around it creating a thickness of about 0.3-0.7mm (Walker).
Nylon 6-6 is chosen to as the predominant thread for the windings because of its strength. Nylon 6-6 has a variety of uses in apparel, but is typically used in products that require high tensile strength and abrasion resistance (“Nylon”). The high tensile strength and abrasion resistance is the reason Nylon adds durability and resistance to deformation for the basketball. Nylon begins its journey in manufacturing with primary materials much like butyl rubber did; crude oil and natural gas. The first step in the process starts with a process called cracking which breaks down larger hydrocarbon molecules into smaller ones, producing benzene and propylene in the process. A series of chemical reactions leads to the final step in which Nylon 6-6 is created from the polycondensation of adipic acid and hexamethylene diamine. It is collected in this raw solid form, which is then heated and melt extruded and spun into Nylon thread.
Polyester is the final major component of the inner windings as well as the basketball. Some basketballs are made using solely Nylon, but polyester can comprise anywhere from 0-40% of the thread used for the carcass. The primary materials used in the reaction that produces polyester is crude oil, coal, water, and air. It is created through a process called polymerization, with the final reaction involving a by product of crude oil, alcohol, as well as carboxyl acid. The polyester is heated and melt extruded like Nylon into thin fibers, which are then spun into Polyester thread.
Once these components have come together to become a basketball, it is time for the latter less material dependent half of the life cycle. Some basketballs are packaged in cardboard exterior boxes to prevent the exterior from abrasion during distribution and sale. Once a basketball has been purchased and is in use, it doesn’t take any additional materials to maintain it. The period of use can vary, but storing the basketball in a room temperature area that is away from sunlight and moisture will maximize its lifespan (“How to Care for a Basketball”). Keeping the ball to the correct pressure is important as well to ensure deformations don’t occur. When a basketball does eventually become unusable due to wear/deformation of the exterior cover or puncture of the rubber bladder, it cannot be recycled. A majority of basketballs end their life cycle in a landfill somewhere, although some are re used in creative ways.
One thing that really stuck out to me about this life cycle was the recurring primary materials of natural gas and crude oil/coal. I initially presumed that fossil fuels would play a part primarily in the energy portion of this life cycle through distribution and manufacture, but I was surprised to find that it played a part in nearly every component. It made it fairly difficult to create a location based life cycle of the fossil fuel based parts of the basketball. Also, synthetic materials such as butyl rubber have numerous reactions required to create them, making it difficult to quantify how much oil was used in production. I emailed ExxonMobil in hopes to get more information about this, but no response was given.
After reviewing the journey of each component of a basketball’s lifecycle independently, it becomes apparent that a product that once seemed simple actually requires an immense amount of materials processing before assembly. Overall, the life cycle of a basketball is very dependent on fossil fuels as a resource for production. The materials associated with the lifecycle are almost exclusively related to raw materials acquisition and manufacturing, as well as some basic materials for distribution. There is a lot of chemical synthesis required just to process the raw materials into the required materials for production. This life cycle demonstrates the complexity associated with even the simplest of products.
Works Cited
"Basketball." How Basketball Is Made - Material, Manufacture, Making, History, Used, Dimensions, Composition, Structure, Steps. N.p., n.d. Web. 24 Nov. 2016. <http://www.madehow.com/Volume-6/Basketball.html>.
Bondy, Filip. "F0LLOW THE BOUNCING BALL Spalding Jumps through Hoops around the World to Stay in Game." NY Daily News. N.p., 24 Mar. 2002. Web. 20 Nov. 2016. <http://www.nydailynews.com/archives/sports/f0llow-bouncing-ball-spalding-jumps-hoops-world-stay-game-article-1.482646>.
"Chromexcel®." Horween Leather Co. N.p., n.d. Web. 28 Nov. 2016. <http://www.horween.com/blog/2010/03/23/chromexcel%C2%AE-2>.
Gerard, Barbara. "How Is Polyester Made?" How Is Polyester Made? Craft Tech Industries, n.d. Web. 23 Nov. 2016. <http://info.craftechind.com/blog/how-is-polyester-made>.
"How to Care for a Basketball." Basketball.epicsports.com. N.p., n.d. Web. 23 Nov. 2016. <http://basketball.epicsports.com/how-to-care-for-a-basketball.html>.
"Nylon." (n.d.): n. pag. Textile Exchange, Jan. 2016. Web. 20 Nov. 1016. <http://textileexchange.org/wp-content/uploads/woocommerce_uploads/2016/03/TE-Material-Snapshot_Nylon-66.pdf>.
"Polyamide 66." Polyamide 66. N.p., n.d. Web. 26 Oct. 2016. <http://pcinylon.com/index.php/markets-covered/polyamide-66>.
"Polyester." How Polyester Is Made - Material, Manufacture, Making, History, Used, Structure, Steps, Product, History. N.p., n.d. Web. 23 Nov. 2016. <http://www.madehow.com/Volume-2/Polyester.html>.
"Rubber Faqs." Rubber Manufacturers Association. N.p., n.d. Web. 18 Nov. 2016. <https://rma.org/about-rma/rubber-faqs>.
Walker, Alan D., Joseph F. Baltronis, and Inc. Lisco. "Patent US5310178 - Basketball with Polyurethane Cover." Google Books. N.p., n.d. Web. 26 Oct. 2016. <https://www.google.com/patents/US5310178>.
Woodford, Chris. "Rubber: A Simple Introduction." Explain That Stuff. N.p., 05 Aug. 2016. Web. 20 Nov. 2016. <http://www.explainthatstuff.com/rubber.html>.
Simon Wu
DES 40A
Christina Cog dell
1 December 2016
Waste & Emissions of a Basketball
Basketball is one of essential modern sporting goods. Most people probably have played basketball during their school age. Professional games, like NBA, are watched all over the world. A basketball, simple enough, consists of a bladder, a carcass and a cover (“Basketball”). However, few may have thought of the waste and emissions coming out of the life cycle a basketball. In this paper, we will specifically look at basketballs from Spalding, the company created the first basketball and currently supplies balls for NBA. While a basketball only has a few components, the materials and process of making one generate more wastes and emissions beyond one’s expectation. Understanding the wastes and emissions involved in making of a basketball can help one to make better eco-friendly purchase decision and encourage the effort to extend the product’s life span.
The acquisition and processing of a baseball’s raw materials produces various wastes. For instance, the production of butyl rubber used in basketball’s bladder produces solid wastes that are unrecyclable and get purged out with stream of water. Butyl rubber is one of many synthesis rubbers and used in a bladder of a Spalding basketball. The production process requires many steps and begins with crude oil in a refinery, and “coal or other hydrocarbons with naphtha as one of the resulting products” (“Production of Synthetic Rubber”). Butyl rubber consists 98% isobutylene and 2% isoprene and its extraction process includes feed blending, polymerization and stripping unreacted monomers, recycle compression and purification, and finishing. During these processes, the main waste is the solvent consists of methyl chloride, unreacted monomers, and hot water used to flash them. Though some solvent and isobutylene are recycled, there are still impurities are purged out, which produces the solid wastes.
Butyl rubber is not the only raw material of a Spalding basketball that generates waste in its production process. Nylon or polyester used in carcass is another example. Nylon, for instance, is associated with greenhouse emission and depletion of ozone. As one of most useful synthetic materials, nylon originates from coal or petroleum, and the most common one is known as nylon 6,6 (Woodford). Threads made from nylon or polyester are used to stitch bladders together to form a ball shape, and “balls used by professional teams have carcasses constructed of nylon…” (“Basketball”). Nylon 6,6 is probably used in making the carcass of Spalding basketballs, since it is most common type of nylon. According to Thiemens and Trogler, nitrous oxide is a by-product of nylon production process and contributes to ozone destruction (932). Besides nylon, polyester is an alternative choice used for threading when making the carcass of Spalding baseballs. Like nylon, polyester is a synthetic fiber that is made from petroleum and “carbon-based nonrenewable resource” (Edwards). Harmful and possibly carcinogenic chemicals are used in the production of polyester, and they can damage the environment through airborne and waterborne wastes if they are untreated. When one plays basketball, he or she probably would never think about that the ball is related to the emission of airborne wastes that can damage the ozone and the potential of producing carcinogenic waste.
Leather, as the cover of a basketball, is the last major material and produce environmental releases depends on whether it is real or synthetic. Spalding provides balls with real leather cover in official NBA games for better indoor performance, whereas average consumers often purchase less expensive balls with synthetic cover for recreational use. Real leather usually comes from ranch animals, like a cow, and the process of produce one fine piece of leather outputs various wastes that threaten the environment (“Environmental Hazards of Leather”). Animals need farmland that produces their foods or pastureland to be fed. Like growing crops, these lands require considerable amount of energy to be maintain job such as tilling and water irrigation. Using fossil fuels as a source of energy is inevitable. Further, tanning process of turning animal skin has its problems, too. It is necessary because it stops the leather from biodegrading, and the effluent from tanning process into leather produces “large amount of pollutants, such as salt, lime sludge, sulfides, and acid” (“Environmental Hazards of Leather”). Even though real leather is from natural material, the wastes from its production should be a factor when one thinks about buying a basketball with a real leather cover.
In addition to real leather, synthetic leather is commonly used in the cover of a basketball as cheaper alternative. While real leather requires large amount of energy input and produces numerous wastes, synthetic leather appears to be more sustainable. Spalding references a patent by Walker and Baltronis’ basketball design, which has polyurethane cover. Hence, polyurethane is hypothetically used for Spalding’s basketballs with synthetic covers for further discussion. According to American Chemistry Council, polyurethanes result from the reaction of a polyol with a diisocyanate, and their versatility and sustainability are emphasized. While little information about waste from its production process, polyurethanes do need various processing equipment for storage and transferring. For example, polyurethanes’ storage tanks can vary from 55-gallon drums to large bulk storage tank farms. The manufacturing of the equipment such as the tanks and transfer process of polyurethanes must require energy input, which probably comes from fossil fuels. The sustainability of polyurethanes is showed in its versatile application and high recyclability. To put into context, polyurethane covers of a decommissioned basketball can be recycled and extend the material usage, whereas real leather is not reusable after it has been worn out. Hence, the wastes generated along the process and recyclability of the materials used in making of a basketball should be considerable when one would like to reduce the environmental impact.
A common concern to synthetic materials is their recyclability and waste management, since they often have low biodegradability. Although synthetic materials used in a basketball produce number of wastes, they are often recyclable. Polyester, for example, is resistant to environmental breakdown, but it is completely recyclable (Edwards). Recycling of these synthetic materials means that some energy and raw materials use can be eliminated. Further, among all the materials used in making of a basketball, the common trend is that they are all require the inputs of fossil fuels, such as petroleum and coal. Some are used as feedstock, and other is used to provide energy needed to manufacture. Either used directly as raw material or indirectly in form of secondary energy, fossil fuels can result in numerous wastes. The extraction, transportation, burning of fossil fuel generate significant amount of emissions and pollutants that are hazardous to the environment and public health ("The Hidden Costs of Fossil Fuels"). The well-known environmental consequence is global warming. The burning of fossil fuels is the primary contributor to carbon dioxide, a primary greenhouse gas. Sulfur dioxide and nitrogen oxides are two examples of air pollutants that are associated with respiratory diseases and smog. Although buying a new basketball may not seem a big deal, if all consumers choose to buy balls that last longer and made with materials that generate fewer wastes and emissions, the sum can make positive impact on the environment.
Though a basketball may not seem an energy-consuming product, millions of basketballs are made, and their production process links to wastes and emissions that can make negative impact on the environment. Since most components of a basketball is made from synthetic materials, many of them can be removed from the waste stream and recycled to reduce the energy required extracting the same material again. Nevertheless, not just basketball, the production of any goods in our daily life requires the input of energy, probably in form burning of fossil fuel. Last length, it’s all about how to use energy more efficiently and reduce amount of energy we need to input to make the same products. Simply giving up the product is not realistic for both consumer’s personal and company’s economic interest. Consumers can pay attention to the products they purchase and choose the ones made from materials generate less wastes and emissions. In the end of a product’s life span, recycling is also a significant step consumers can take to reduce the energy consumption of extracting the raw materials. Perhaps, like what Ozzie Zehner said in his book Green Illusions, “the best material consumption is less material consumption.” If everyone on the planet can reduce his or her consumption, fewer wastes and emissions will be released to our threatened environment.
Bibliography
"Environmental Hazards of Leather." PETA. N.p., n.d. Web. 16 Nov. 2016. <http://www.peta.org/issues/animals-used-for-clothing/leather-industry/leather-environmental-hazards/>.
"Polyurethanes." How Polyurethane Is Made. American Chemistry Council, n.d. Web. 23 Nov. 2016. <https://polyurethane.americanchemistry.com/How-Polyurethane-is-Made/>
"The Hidden Costs of Fossil Fuels." Union of Concerned Scientists. N.p., n.d. Web. 21 Nov. 2016. <http://www.ucsusa.org/clean-energy/coal-and-other-fossil-fuels/hidden-cost-of-fossils#.WDO7pXc-JTY>.
“Basketball.” How Basketball Is Made. Advameg, Inc., 2016. Web. 24 Oct. 2016. <http://www.madehow.com/Volume-6/Basketball.html>.
“History - Spalding.” Spalding Basketball. Spalding, 2016. Web. 15 Nov. 2016. <http://www.spalding-basketball.com/en/about-spalding/history/>.
“Production of Synthetic Rubber.” Simens Process Analytics. Jan 2013. <https://w3.siemens.com/mcms/sensor-systems/CaseStudies/CS_Butyl_Rubber_2013-01_en_Web.pdf>.
Edwards, Summer. "Why Polyester Production Damages the Environment." Peaceful Dumpling. N.p., 19 Sept. 2016. Web. 22 Nov. 2016. <http://www.peacefuldumpling.com/why-polyester-production-damages-the-environment>.
Thiemens, Mark H., and William C. Trogler. "Nylon production. An unknown source of atmospheric nitrous oxide." Science(Washington) 251.4996 (1991): 932-934.
Walker, Alan D., and Joseph F. Baltronis. "Basketball with polyurethane cover." U.S. Patent No. 5,310,178. 10 May 1994.
Woodford, Chris. "Nylon - The Science of Synthetic Textiles." Explain That Stuff. N.p., 18 June 2016. Web. 22 Nov. 2016. <http://www.explainthatstuff.com/nylon.html>.
Zehner, Ozzie. Green Illusions: The Dirty Secrets of Clean Energy and the Future of Environmentalism. Lincoln: U of Nebraska, 2012. Print.