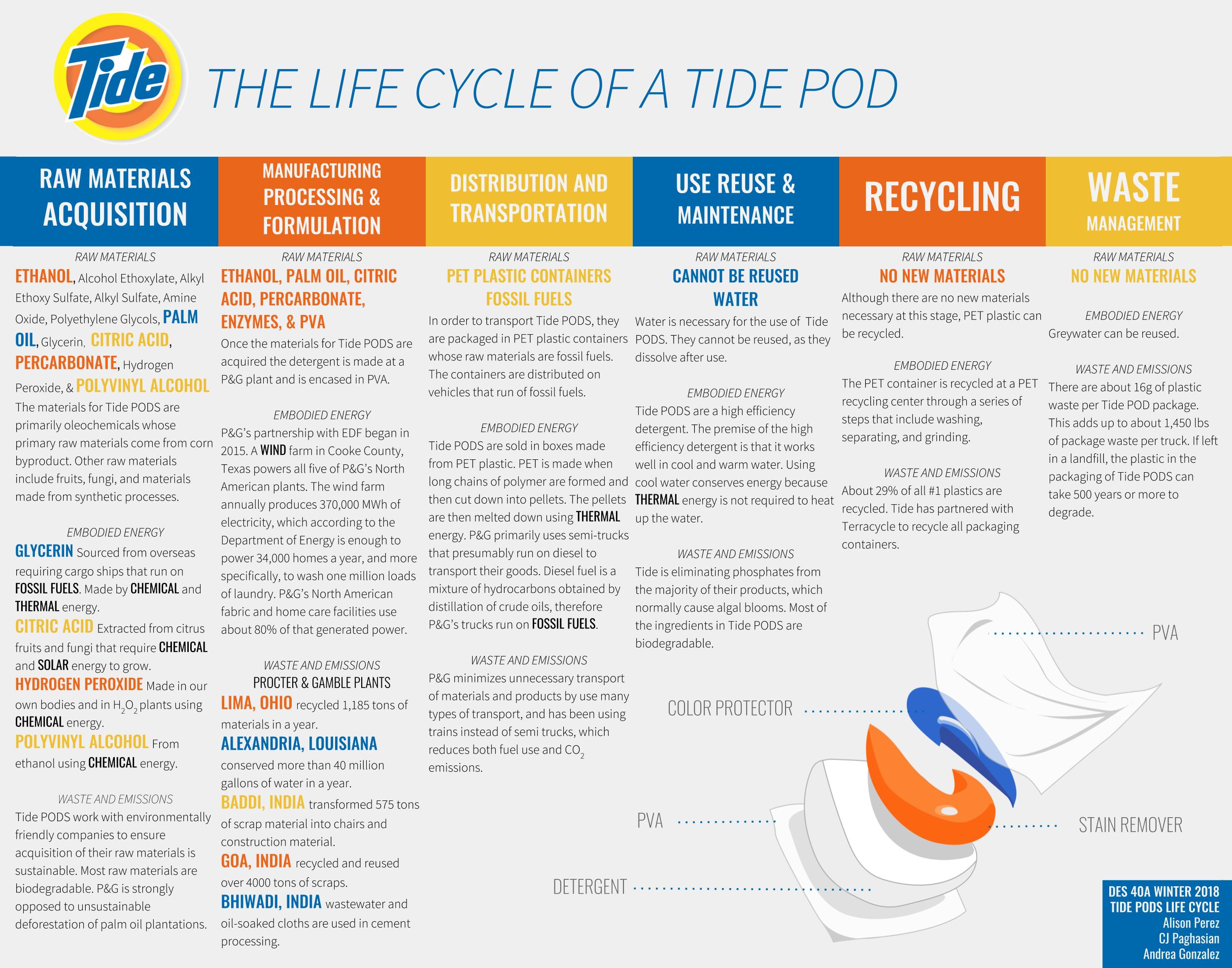
Design Life-Cycle
assess.design.(don't)consume
Andrea Gonzalez
Christina Cogdell
DES 40A A01
15 March 2018
The Raw Materials in a Tide PODS Life Cycle
Introduction
The 1950’s brought affordable top loading machines to American homes, and with each top loading came a cardboard box of Tide detergent powder[1]. That was how Tide, the first heavy-duty synthetic clothing detergent, was introduced to the market[2]. Over time, Tide has reformulated their laundry detergents to be more efficient and convenient for their consumers. In 2012, Tide introduced Tide PODS to the market, further facilitating doing laundry.
As of recently, Tide PODS have caught a lot of media attention due to the infamous Tide PODS challenge, where teens ingest the detergent pacs[4]. This challenge arose due to Tide PODS’ unique packaging; the colorful detergent is packed inside of a water soluble film, giving it a candy-like appearance. Although the pacs have become a danger to the public, they are an improvement on laundry detergents currently in the market. Tide PODS are advertised as having 10 times the cleaning power of the leading laundry detergent, being able to remove stains, protecting color, and dissolving in all temperatures[3]. The formulation Tide PODS are what has made them an improvement for consumers and through the assessment of the raw materials, it is clear that Tide is moving toward becoming more efficient and sustainable.
Raw Materials Acquisition
Tide PODS are listed as having a variety of ingredients. The ingredients in Tide PODS are: Alcohol Ethoxylate, Alkyl Ethoxy Sulfate and Alkyl Sulfate, Citric Acid, Ethanol, Hydrogen Peroxide, Percarbonate, Polyethylene Glycols (PEG), and Polyvinyl Alcohol[5]. I have come to the realization that these ingredients are not the raw materials for Tide PODS, but rather secondary or even tertiary materials. While they are not raw materials, the purest ingredients listed for Tide PODS are Citric Acid, Ethanol, and Percarbonate. Some of the ingredients used for Tide PODS, I have assumed, are derived from these main ingredients. As for other ingredients, I could not determine where they were derived from.
Ethanol & Its Derivatives
One of the secondary raw materials in Tide PODS is Ethanol. It is a colorless alcohol that is used as a solvent in Tide detergents [5]. Ethanol is an oleochemical that is produced by Procter and Gamble(P&G), the company who owns Tide. Oleochemicals are substances derived from vegetable or animal fat[6]. The primary raw material that P&G uses in order to produce ethanol is corn byproduct. I do not know where the corn byproducts are sourced, but it is likely that they come from the United States or overseas from Asian countries. I have not been able to find the process that P&G uses to create ethanol, but in order to turn corn byproduct into alcohol, it must go through dry milling or wet milling[7]. Both processes involve the extraction of oils which then undergo fermentation[8]. Corn byproducts were not always the primary raw material that P&G used for ethanol. The use of corn byproducts, according to P&G, will produce fewer greenhouse emissions[9]. This change is an effort from P&G to become a more sustainable company.
Other alcohol based oleochemicals that are used in the Tide PODS detergent are surfactants. Surfactants are surface-active agents that bind to clothing to remove stains[10]. The surfactants in the Tide PODS detergent are: Alcohol Ethoxylate, Alkyl Ethoxy Sulfate and Alkyl Sulfate, and Polyethylene Glycols. Alcohol Ethoxylate is produced through the ethoxylation of primary alcohols[9]. In order to ethoxylate alcohol, ethylene oxide must be added with a base like potassium or sodium hydroxide[11]. Then, the compound is neutralised with an acid like phosphoric acid[11]. P&G tells us that the alcohol used for the ethoxylation process is derived from vegetable oil[12]. Due to being able to produce ethanol from corn byproducts, I have assumed that one of the primary raw materials for Alcohol Ethoxylate is also corn byproduct.
Another surfactant used in the Tide PODS detergent is Alkyl Ethoxy Sulfate and Alkyl Sulfate. These surfactants, like Alcohol Ethoxylate, are oleochemicals that Procter and Gamble produces. Because they are produced by Procter and Gamble, I have assumed that the primary raw materials for Alkyl Ethoxy Sulfate and Alkyl Sulfate are corn byproducts. Oils are extracted from the corn byproduct in order to produce ethanol through fermentation. The ethanol is then ethoxylated, most likely with ethylene oxide[11]. The Alcohol Ethoxylate is then sulphanized with sulphur trioxide or chlorosulphonic acid[13].
The last surfactant in the Tide PODS detergent is Polyethylene Glycol[5]. This ingredient is a polyether compound, meaning that it is an organic compound with molecules that are synthesized into polymers. The main ingredient for polyethylene glycol is methyl ether[14]. Procter and Gamble’s methyl ether is manufactured in Sacramento, California and the raw materials for it are coconut and palm kernel oils[14]. These raw materials come from Asia, Europe and North America[14]. In order to produce Polyethylene Glycol, the methyl ether and water are pressurized[15].
Glycerin
Glycerin is another ingredient within the Tide PODS detergent. P&G’s glycerin is produced in North America and is derived from vegetable oils[16]. It is not clear what the vegetable oil for the glycerin is. I have assumed that the material for glycerin is palm oil, making the primary raw material mesocarp. In order to make glycerin from palm oil, the fats and oils must be split from each other with the use of water[17]. It is not clear where the palm oil is sourced from, but it is likely that it comes overseas from Malaysia or Indonesia and is manufactured into glycerin in the United States[16]. This is troubling because the palm oil industry is responsible for a lot of deforestation in these parts of the world. P&G states that the traceability of their palm oil is important in order to assure that there are sustainable practices that will not lead to further deforestation[18].
Citric Acid
Citric Acid is another ingredient used in the Tide PODS detergent. When used in detergent, citric acid acts as a chelating agent[5]. It can be derived naturally in two ways. Citric acid is produced naturally within citrus fruits. Although it can be produced within fruits, Citric acid is produced naturally from Aspergillus Niger when it is fermented[19]. It it not clear how or where the Citric Acid for the detergent is obtained. It is more likely that P&G produces their citric acid through Aspergillus Niger and not actual citrus fruits, as it is more cost effective.
Percarbonate & Hydrogen Peroxide
Percarbonate and Hydrogen Peroxide are also used for the Tide PODS detergent. They are both common bleaching agents in detergents[5]. Percarbonate is synthesized soda ash and Hydrogen Peroxide[20]. Soda ash can be sourced from minerals within the Earth, and hydrogen peroxide be produced naturally or synthetically. Hydrogen peroxide, when produced synthetically, begins with the hydrogenation of anthraquinone with hydrogen[21]. The solution is then oxidized with air[21]. Once oxidized, hydrogen peroxide is extracted with water[21]. It is not very clear where the raw materials for this process come from or where they are produced, but most of the hydrogen peroxide today is produced in China. Polyvinyl Alcohol
The colorless film that encases the Tide PODS detergent is Polyvinyl Alcohol. Polyvinyl Alcohol is soluble in water which makes it suitable for encasing the detergent[5]. The raw material for Polyvinyl Alcohol is ethanol. As previously mentioned, it is likely that P&G produces ethanol through the dry or wet milling of corn byproduct which might be sourced from the United States or Asia.[7]. Then, acetic acid is used to produce vinyl acetate that is turned into a polymer[22]. It is not clear how the vinyl acetate is polymerized, but once polymerized, it becomes water soluble when dissolved in alcohol[22].
Manufacturing Processing & Formulation
The Tide PODS detergent is made with Ethanol, Alcohol Ethoxylate, Alkyl Ethoxy Sulfate and Alkyl Sulfate, Polyethylene Glycols, Glycerin, Citric Acid, Percarbonate, and Hydrogen Peroxide[5]. The detergent is made in Procter and Gamble’s plants which are located in the United States and India. In order to power the plants where the detergent is manufactured, P&G partnered with EDF to create a wind farm that generates enough energy to power their plants in the United States and India. P&G has made the switch to renewable energy in efforts to reduce greenhouse gas emissions by 30% by 2020[23].
The materials needed are transported to these locations, most likely on trucks, ships or planes. The transportation vehicles most likely use fossil fuels, which come naturally from the Earth. Once these materials are acquired, I presume that there is a process that P&G uses in order to manufacture their detergent. Once the detergent is made, it goes through a machine that is lined with Polyvinyl Alcohol film. The detergent is placed on the film and it is sealed in order to prevent spilling. After This process, the Tide PODS are ready for use.
Distribution and Transportation
Once the Tide PODS are manufactured, they are packaged and transported to different locations where they are purchased. The packaging for Tide PODS is a plastic container made of PET plastic. The primary materials for PET plastic are derived from crude oils and natural gas[24]. The raw materials are turned to plastics through a polymerization process[24]. Once the fossil fuels are turned to plastic, it is likely that the plastic pellets are melted and moulded or blown into a container. These containers are then transported to stores with trucks that run on fossil fuels. Fossil fuels form within the Earth over long periods of time[25]. They form due to phytoplankton that died and photosynthesized with the sun's energy to form shale rock[25]. Fossil fuels come from many parts of the world, most commonly in Saudi Arabia, Russia, and the United States.
Use, Reuse, & Maintenance
In order to use Tide PODS, a laundry machine with running water is needed. The water is needed to dissolve the Polyvinyl Alcohol film. At this point, the user is responsible for the water source, so it is difficult to determine where the water is coming from. The electricity needed to power the washing machine is also determined by the user. Once the Tide PODS are used to do laundry, they cannot be reused. There is no maintenance needed for the Tide PODS; the user simply goes to the store to purchase another container of Tide PODS once they run out.
Recycling & Waste Management
At this point in the life cycle, the Tide PODS have dissolved, their life has ended and cannot be recycled. Although Tide PODS cannot be recycled, the PET plastic container in which they are packaged can be recycled. PET plastic can be put into the recycling bin, or taken to a nearby recycling facility. When the plastic reaches a recycling facility, the plastic is melted and can be turned into new plastic or synthetic fibers[26]. It is unclear what raw material is used to power recycling facilities, as they may run on renewable or non renewable energy. I have assumed however that they are most likely powered with fossil fuels. The water that is used to do laundry leaves the washing machine as greywater[27]. The greywater can be collected by the user to water their landscape. The materials for this process are chosen by the user, but it is likely that the water is collected in a plastic container. If the water goes down the drain, I would assume that it goes through other systems where it could be treated and reused[26]. It is not clear how the water is treated, but I presume that it requires many chemicals.
Conclusion
In short, Procter and Gamble is making efforts toward creating an efficient and sustainable future. P&G is transparent about most of the materials needed for Tide PODS. The change to corn byproducts to make ethanol and switch to wind power are notable changes that P&G has made in order to reduce greenhouse gases. Although P&G is working toward a more sustainable future, some raw materials and distribution methods still require materials like Palm Oil and and fossil fuels. Despite the use of these materials, P&G practices more sustainable methods in the acquisition and use of these materials, and will continue to improve upon their Tide products like they have in the past.
Works Cited
[1] "About Us." History | Learn About Tide the Brand - Tide. tide.com/en-us/about-tide/about-us.
[2] American Chemical Society. The Development of Tide. American Chemical Society, 2006.
[3] Bever, Lindsey. “Teens Are Daring Each Other to Eat Tide Pods. We Don't Need to Tell You
That's a Bad Idea.” The Washington Post, WP Company, 17 Jan. 2018, www.washingtonpost.com/news/to-your-health/wp/2018/01/13/teens-are-daring-each-other-to-eat-tide-pods-we-dont-need-to-tell-you-thats-a-bad-idea/?utm_term=.0e62a58b42d.
[4] "Laundry Detergent Pacs. | Tide PODS®." Laundry Detergent Pacs | Tide PODS® Original. 18 Feb. 2018. Web.
[5] “Laundrypedia; Laundry Detergent Ingredients Explained!” Laundrypedia; Detergent
Ingredients | Innovation - Tide, tide.com/en-us/about-tide/innovation/detergent-ingredients.
[6] “Oleochemical.” Oleochemical Dictionary Definition | Oleochemical Defined,
www.yourdictionary.com/oleochemical.
[7] Aithani, Dinesh, and Amar K. Mohanty. “Value-Added New Materials from Byproduct of
Corn Based Ethanol Industries: Blends of Plasticized Corn Gluten Meal and Poly(ε-Caprolactone).” Industrial & Engineering Chemistry Research, vol. 45, no. 18, 2006, pp. 6147–6152., doi:10.1021/ie0513200.
[8] Randhava Sarabjit S, Kao Richard L, Calderone Steven G, and Randhava Ajaib S. (2008).
https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=1&ND=3&adjacent=true&FT=D&date=20080724&CC=US&NR=2008176298A1&KC=A1.
[9] “Supplier Efforts.” Sustainability | P&G,
us.pg.com/sustainability/environmental-sustainability/supplier-efforts.
[10] Britannica, The Editors of Encyclopaedia. “Surfactant.” Encyclopædia Britannica,
Encyclopædia Britannica, Inc., 10 Mar. 2016, www.britannica.com/science/surfa
[11]Plata, María R., et al. “Analytical Characterization of Alcohol-Ethoxylate Substances by
Instrumental Separation Techniques.” TrAC Trends in Analytical Chemistry, vol. 30, no. 7, 2011, pp. 1018–1034., doi:10.1016/j.trac.2011.02.015.
[12] “Fatty Alcohol Ethoxylate Suppliers.” P&G Chemicals,
www.pgchemicals.com/oleochemicals-products/alcohol-ethoxylates.
[13] Sibila, M.A., et al. “Ecotoxicity and Biodegradability of an Alkyl Ethoxysulphate Surfactant
in Coastal Waters.” Science of The Total Environment, vol. 394, no. 2-3, 2008, pp. 265–274., doi:10.1016/j.scitotenv.2008.01.043.
[14] “Methyl Ester Producers – Coconut & Palm Kernel Oils.” P&G Chemicals,
www.pgchemicals.com/oleochemicals-products/methyl-esters.
[15] “Polyethylene Glycol.” Polyethylene Glycol - an Overview | ScienceDirect Topics,
www.sciencedirect.com/topics/neuroscience/polyethylene-glycol.
[16] “Glycerin Suppliers & Producers.” P&G Chemicals,
www.pgchemicals.com/oleochemicals-products/glycerin.aspx.
[17] A Method of Rapid Fat and Oil Splitting Using a Lipase Catalyst Found in Seeds : USDA
ARS, www.ars.usda.gov/northeast-area/wyndmoor-pa/eastern-regional-research-center/docs/technology-transfer/a-method-of-rapid-fat-and-oil-splitting-using-a-lipase-catalyst-found-in-seeds/.
[18] “PG.com Renewable Resources: Sustainably Sourced Renewable Materials.” PG.com
Sustainability: Sustainably Sourced Renewable Materials, www.pg.com/en_US/sustainability/environmental_sustainability/renewable_resources/renewable_resources.shtml.
[19] Karaffa, Levente, and Christian P. Kubicek. “Aspergillus Niger Citric Acid Accumulation:
Do We Understand This Well Working Black Box?” Applied Microbiology and Biotechnology, vol. 61, no. 3, 2003, pp. 189–196., doi:10.1007/s00253-002-1201-7.
[20] Guo, Zitian, et al. “In Situ Synthesis of Solid Base Catalysts for the Regeneration of
Degradation Products Formed during the Anthraquinone Process for the Manufacture of Hydrogen Peroxide.” Applied Catalysis A: General, vol. 401, no. 1-2, 2011, pp. 163–169., doi:10.1016/j.apcata.2011.05.013.
[21] Khanmohammadi, Mohammadreza, et al. “Quantitative Determination of Sodium Perborate
and Sodium Percarbonate in Detergent Powders by Infrared Spectrometry.” Journal of Analytical Chemistry, vol. 67, no. 4, 2012, pp. 330–334., doi:10.1134/s1061934812040089.
[22] Kobayashi, Shiro, and K. Müllen. “Encyclopedia of Polymeric Nanomaterials.” 2015,
doi:10.1007/978-3-642-29648-2.
[23] “Texas Wind Farm to Generate 370,000 MWh of Electricity a Year.” EDF Renewable
Energy, 5 Oct. 2016, www.edf-re.com/procter-gamble-make-iconic-brands-including-tide-dawn-wind-power/.
[24]“An Introduction to PET.” Fact Sheet - An Introduction to PET (Polyethylene Terephthalate)
| PETRA: Information on the Use, Benefits & Safety of PET Plastic., www.petresin.org/news_introtoPET.asp.
[25] Nance, Damian, and Brendan E. Murphy. Physical Geology Today. Oxford University Press,
2016.
[26] Winter, Debra. “The Violent Afterlife of a Recycled Plastic Bottle.” The Atlantic, Atlantic
Media Company, 4 Dec. 2015, www.theatlantic.com/technology/archive/2015/12/what-actually-happens-to-a-recycled-plastic-bottle/418326/.
[27] Wastewater Recycling | Sustainability Workshop,
sustainabilityworkshop.autodesk.com/buildings/wastewater-recycling.
Bibliography
A Method of Rapid Fat and Oil Splitting Using a Lipase Catalyst Found in Seeds : USDA ARS, www.ars.usda.gov/northeast-area/wyndmoor-pa/eastern-regional-research-center/docs/technology-transfer/a-method-of-rapid-fat-and-oil-splitting-using-a-lipase-catalyst-found-in-seeds/.
“About Us.” History | Learn About Tide the Brand - Tide, tide.com/en-us/about-tide/about-us.
Aithani, Dinesh, and Amar K. Mohanty. “Value-Added New Materials from Byproduct of Corn Based Ethanol Industries: Blends of Plasticized Corn Gluten Meal and Poly(ε-Caprolactone).” Industrial & Engineering Chemistry Research, vol. 45, no. 18, 2006, pp. 6147–6152., doi:10.1021/ie0513200.
“An Introduction to PET.” Fact Sheet - An Introduction to PET (Polyethylene Terephthalate) | PETRA: Information on the Use, Benefits & Safety of PET Plastic., www.petresin.org/news_introtoPET.asp.
Bever, Lindsey. “Teens Are Daring Each Other to Eat Tide Pods. We Don't Need to Tell You That's a Bad Idea.” The Washington Post, WP Company, 17 Jan. 2018, www.washingtonpost.com/news/to-your-health/wp/2018/01/13/teens-are-daring-each-other-to-eat-tide-pods-we-dont-need-to-tell-you-thats-a-bad-idea/?utm_term=.0e62a58b42d5.
Britannica, The Editors of Encyclopaedia. “Surfactant.” Encyclopædia Britannica, Encyclopædia Britannica, Inc., 10 Mar. 2016, www.britannica.com/science/surfactant.
“Encyclopedia of Polymeric Nanomaterials.” 2015, doi:10.1007/978-3-642-29648-2.
“Fatty Alcohol Ethoxylate Suppliers.” P&G Chemicals, www.pgchemicals.com/oleochemicals-products/alcohol-ethoxylates.
“Glycerin Suppliers & Producers.” P&G Chemicals, www.pgchemicals.com/oleochemicals-products/glycerin.aspx.
Guo, Zitian, et al. “In Situ Synthesis of Solid Base Catalysts for the Regeneration of Degradation Products Formed during the Anthraquinone Process for the Manufacture of Hydrogen Peroxide.” Applied Catalysis A: General, vol. 401, no. 1-2, 2011, pp. 163–169., doi:10.1016/j.apcata.2011.05.013.
Karaffa, Levente, and Christian P. Kubicek. “Aspergillus Niger Citric Acid Accumulation: Do We Understand This Well Working Black Box?” Applied Microbiology and Biotechnology, vol. 61, no. 3, 2003, pp. 189–196., doi:10.1007/s00253-002-1201-7.
Khanmohammadi, Mohammadreza, et al. “Quantitative Determination of Sodium Perborate and Sodium Percarbonate in Detergent Powders by Infrared Spectrometry.” Journal of Analytical Chemistry, vol. 67, no. 4, 2012, pp. 330–334., doi:10.1134/s1061934812040089.
“Laundry Detergent Pacs | Tide PODS®.” Laundry Detergent Pacs | Tide PODS® Original, 18 Feb. 2018, tide.com/en-us/shop/type/laundry-pods/tide-pods-laundry-detergent#loadmore.
“Laundrypedia; Laundry Detergent Ingredients Explained!” Laundrypedia; Detergent Ingredients | Innovation - Tide, tide.com/en-us/about-tide/innovation/detergent-ingredients.
“Methyl Ester Producers – Coconut & Palm Kernel Oils.” P&G Chemicals, www.pgchemicals.com/oleochemicals-products/methyl-esters.
Nance, Damian, and Brendan E. Murphy. Physical Geology Today. Oxford University Press, 2016.
“Oleochemical.” Oleochemical Dictionary Definition | Oleochemical Defined, www.yourdictionary.com/oleochemical.
“PG.com Renewable Resources: Sustainably Sourced Renewable Materials.” PG.com Sustainability: Sustainably Sourced Renewable Materials, www.pg.com/en_US/sustainability/environmental_sustainability/renewable_resources/renewable_resources.shtml.
Plata, María R., et al. “Analytical Characterization of Alcohol-Ethoxylate Substances by Instrumental Separation Techniques.” TrAC Trends in Analytical Chemistry, vol. 30, no. 7, 2011, pp. 1018–1034., doi:10.1016/j.trac.2011.02.015.
“Polyethylene Glycol.” Polyethylene Glycol - an Overview | ScienceDirect Topics, www.sciencedirect.com/topics/neuroscience/polyethylene-glycol.
Randhava Sarabjit S, Kao Richard L, Calderone Steven G, and Randhava Ajaib S. (2008).
Sibila, M.A., et al. “Ecotoxicity and Biodegradability of an Alkyl Ethoxysulphate Surfactant in Coastal Waters.” Science of The Total Environment, vol. 394, no. 2-3, 2008, pp. 265–274., doi:10.1016/j.scitotenv.2008.01.043.
“Supplier Efforts.” Sustainability | P&G, us.pg.com/sustainability/environmental-sustainability/supplier-efforts.
“Texas Wind Farm to Generate 370,000 MWh of Electricity a Year.” EDF Renewable Energy, 5 Oct. 2016, www.edf-re.com/procter-gamble-make-iconic-brands-including-tide-dawn-wind-power/.
Wastewater Recycling | Sustainability Workshop, sustainabilityworkshop.autodesk.com/buildings/wastewater-recycling.
Winter, Debra. “The Violent Afterlife of a Recycled Plastic Bottle.” The Atlantic, Atlantic Media Company, 4 Dec. 2015, www.theatlantic.com/technology/archive/2015/12/what-actually-happens-to-a-recycled-plastic-bottle/418326/.
Alison Perez
Christina Cogdell
15 March 2018
DES 40A Winter 2018
Wash, Dry, Fold: A Look at the Energy in the Lifecycle of Tide PODS®
Introduction
Let us face the facts: our lives have been enhanced by products and gadgets that make everyday life easy. We welcome these things because of their prospect to upgrade, even slightly, certain aspects of living. We try them, and continue to use them, yet we do not question what is in them, how they are made, how they get to us, or where they go after they are used. Those phases are invisible, and we do not care as long as the product works for us. Sure, something can work well for us in the short term, but those core processes can affect our lives in the long term.
One product many people use on a regular basis are Tide PODS®, those small, bright-colored, square “pillows” that revolutionize the way we do laundry. These are laundry pacs that are made up of a “highly concentrated detergent, stain remover and color protector” and are used in place of regular detergent, just throw a couple in with your load [i]. Tide, manufactured by Procter and Gamble (P&G), is a well-known label, and according to the Tide website, “more than 40 million American households used Tide laundry detergent in 2014-2015, and in terms of sales, Tide is the country's number one detergent” [ii]. With a widely used and popular brand, it is crucial for P&G’s consumers to know exactly how they operate. Researching the embodied energy in the life cycle of Tide PODS® allows us to examine how P&G finds alternative and environmentally friendly ways to acquire raw materials, manufacture and distribute the products, and recycle and manage waste.
Raw Materials Acquisition
At the forefront of any environmentally friendly product is ensuring that the raw materials used in its production are as such. A number of ingredients goes into Tide PODS®, some of these include citric acid, glycerin, hydrogen peroxide, and polyvinyl alcohol.
Citric acid is used to remove odors in clothes and as a chelating agent, a water softener. In nature, citric acid can be found in citrus fruits. Citrus fruit-bearing trees require an abundance of solar energy, and in turn chemical energy is used to perform photosynthesis. The process of extracting citric acid is chemical energy at work. When lemon juice is mixed with calcium hydroxide, the solution turns to calcium citrate. Add sulfuric acid to the calcium citrate, and the solution becomes citric acid [iii]. Researchers discovered that certain molds, fungi, and yeasts can also produce citric acid during fermentation. This discovery led to the cultivation of citric acid on a cost-effective large scale [iv]. Citric acid is produced in organisms through the Krebs cycle, also known as the citric acid cycle. The citric acid cycle is the chemical process that converts oxygen into CO2, with the main byproduct of the cycle being citric acid.
Glycerin has its presence in all P&G products, including detergents. Glycerin is found in many soaps and is known as a humectant, a substance that retains moisture. Also known as glycerol, it is an organic compound found in animal fat or vegetable oils. Making glycerin from animal fats and vegetable oils is a matter of melting them down with thermal energy and using chemical energy when lye and salt is added [vi]. Glycerin is produced by refining crude glycerin through a series of purification steps. Crude glycerin is derived through consumption of chemical energy when the glycerin chain is split from the triglyceride backbone [vii]. P&G has been producing glycerin for nearly as long as their products have been produced, and today P&G is one of the leading suppliers in the world’s glycerin [v]. Most of P&G’s glycerin is sourced within the Americas, and only a fraction of it is sourced from Southeast Asia. Receiving glycerin from overseas requires shipping, and cargo ships primarily run on oil and various other fossil fuels. Cargo ships convert the chemical energy in oil and fuel into mechanical or kinetic energy.
Hydrogen peroxide (H2O2) is a simple and common bleaching agent. As an oxidizing agent, it has an array of uses, from first aid to household cleaning. H2O2 can actually be made in our own bodies using chemical energy when it is created by white blood cells in the thyroid, gut, and lungs to supply our immune system with this oxygen free radical to destroy harmful bacteria, fungi, and viruses [viii]. Scientists found that when the tails of zebrafish are injured, H2O2 is released from the wound, enters the surrounding tissue, and white blood cells respond to the chemical signal and repair the damage all within a few minutes [ix]. However, H2O2 which we find in brown bottles in our medicine cabinets and in laundry detergents is made on a much larger scale and is made primarily using chemical energy. 99% of the world’s hydrogen peroxide is manufactured by an autoxidation process, and it begins with the hydrogenation of anthraquinone, then the oxidation of the resulting anthraquinol. It is finished with the extraction of the H2O2 solution by adding water, and then the purification and concentration of the H2O2. In 2015, the world’s production of hydrogen peroxide reached 4.3 million tons, with China producing 1.5 million of that and the US producing 400,000 [x].
Arguably the most important feature of the Tide POD is the magical casing that disappears when thrown into the wash. The casing is made of a water-soluble synthetic polymer called polyvinyl alcohol (PVA). PVA is formed using chemical energy and presumably fossil fuels for various machinery in the production of synthetic ethanol. It starts as ethylene, an important plant hormone responsible for fruit ripening. P&G and DuPont, a chemical company, collaborate to use cellulosic ethanol made from chemical energy through the fermentation and distillation of corn [xi]. After ethanol is made synthetically from hydration of ethylene, it is turned into vinyl acetate through a chemical reaction with acetic acid and oxygen. It is then polymerized and broken down in alcohol to give it its signature water-soluble quality [xii].
Manufacturing, Processing, Formulation
Tide has several factories throughout the US, but most noteworthy are the plants in Lima, Ohio and Alexandria, Louisiana. The Lima plant has conserved enough oil to heat and cool approximately 2,600 homes for one year and enough gasoline to drive over 763,000 miles. The water saved can meet the needs of 110,000 Americans and enough kilowatt-hours (kWh) of energy to power 388 homes for a year. The plant in Alexandria conserved over 40 million gallons of water over the course of a year and 24 million kWh of electricity [xiii]. These feats result from P&G’s partnership with EDF Renewable Energy which began in 2015. Since a wind farm in Cooke County, Texas dedicated to P&G was built and fully operational by December 2016, P&G relied on wind to supply power to all five of its North American plants. The wind farm in Cooke County annually produces 370,000 megawatt-hours of electricity which, according to the Department of Energy, is enough to power 34,000 homes a year, or more specifically, to wash one million loads of laundry. P&G’s North American fabric and home care facilities use about 80% of that generated power. The switch to renewable energy started with the American Business Act on Climate Pledge, and through the pledge P&G has agreed to reduce its greenhouse gas emissions 30% by 2020 [xiv] [xv].
Researching the manufacturing process of Tide PODS® proved difficult. However, there are several videos on the internet showing how cleaning pods are made. In general, laundry and dishwasher pods are made using a rotating machine with indents in the shape of the pods. PVA is pressed into the indents, which are then filled with the liquid, then sealed with another layer of PVA. The sheets of pods are cut by another machine and then packaged. Now that we know Tide’s plants are powered by wind power, it is safe to say that wind power is converted into mechanical energy that powers the machinery within the plants [xvi].
Distribution and Transportation
Tide PODS® are sold in plastic bags and lidded plastic containers labeled with recycling code #1, PET/PETE plastic. PET stands for polyethylene terephthalate, a strong plastic belonging to the polyester family. It is a common choice for food and beverage packagings because it is hygienic, strong, lightweight, shatterproof, and retains freshness, all important qualities for the Tide PODS® box. PET is made when ethylene glycol and terephthalic acid are combined under high temperatures and low vacuum pressure. Long chains of polymer are formed and cut down into pellets. The pellets are melted down using thermal energy [xvii].
For distribution of packaged Tide PODS®, P&G primarily uses semi-trucks that presumably run on diesel. Diesel fuel is a mixture of hydrocarbons obtained by distillation of crude oils, therefore P&G’s trucks run on fossil fuels [xviii]. However, according to the P&G website, their 2020 goal is to reduce truck transportation kilometers by 20% per unit of production versus their 2010 baseline. This goal was reached and exceeded in 2015. Truck transportation was reduced by 25% by improving vehicle fill rate, optimization of distribution routes and driving, and increased use of different forms of transportation [xix].
Use, Re-Use, Maintenance
Why are Tide PODS® a revolutionary product? One reason is their incredible ease of use. They are thrown into the wash without manual measuring so nearly no human energy is exerted. Another reason is their concentrated formula and high efficiency, the meaning of the “HE” notation on the packaging. The whole premise of the high efficiency detergent is that it works just as well in cool as it does in warm water. Using cool water conserves energy because it eliminates the use of thermal energy to heat up the water. HE detergents also give 6x better clean in half the wash time. They cut water usage in half because they do not produce as many suds [xx]. By offering more concentrated formulas, Tide is taking a major step in reducing their overall environmental impact. Tide PODS®, being the most concentrated, provide the same number of loads but in smaller and lighter package sizes, resulting in less packaging waste (primary and secondary), and by helping to reduce emissions from delivery vehicles during transportation due to lighter packages [xxi].
Recycling
The only thing that could be recycled is the Tide PODS® packaging. PET/PETE plastic is easy to recycle. PET is sorted from other recyclables and delivered by truck, to a PET recycling facility where the process begins with separating the bales of plastic on a conveyor belt. Water is used to clean the plastics. They are run through a machine that removes and separates labels and loose caps by using steam and other chemicals. The process continues with multiple cycles of washing and metal detecting. The PET is ground into flakes and washed with hot water and chemicals to remove dirt and glue. The flakes are rinsed and spun dry and then sent to a blender where residual PVC is detected and separated using an elutriator. The PET is held in a storage silo until ready to be cleaned for use in food packaging [xxii].
Waste Management
Once Tide PODS® are used, not much happens afterwards. It dissolves in the load, then the greywater is drained. Greywater is the gently used waste water from showers, baths, washing machines, dishwasher, etc. Greywater is generally stored in our septic systems when it goes down the drain, but it is up to the user if and how they want to recycle their greywater before it goes down the drain. There is no information about the greywater from Tide PODS® being particularly harmful, since it would be like any other detergent or soap product’s greywater.
Conclusion
The amount of energy that goes into creating a Tide POD should not be overlooked. It is a multistep process to gather raw materials, combine those raw materials, package and deliver the product to consumers. Most companies do not take into account their impact on the environment when they are making a product they can sell for profit. However, through evaluating the embodied energy of the lifecycle of a Tide POD, it is safe to say that Tide and its manufacturer Procter & Gamble are making strides in becoming a forerunner for eco-friendly production processes and innovative technology for the consumer.
Works Cited
[i] "Laundry Detergent Pacs | Tide PODS®." Laundry Detergent Pacs | Tide PODS® Original. Web.
[ii] "The Results Are In: Tide Is America's #1 Detergent." America's #1 Laundry Detergent
Tide. Web.
[iii] Whitehouse, Jordan. "How to Extract the Citric Acid From Lemons." LEAFtv. Web.
[iv] Busch, Sandi. "How Is Citric Acid Made & Where Does It Come From?"
LIVESTRONG.COM. Leaf Group, 03 Oct. 2017. Web.
[v] "Glycerin Suppliers & Producers." P&G Chemicals. Web.
[vi] J, Michael. "How to Make Glycerin From Vegetable Oil." Sciencing. Leaf Group, 03 June
2017. Web.
[vii] P&G Chemicals, “Superol KPO Kosher, USP/FCC/EP,” technical datasheet.
[viii] "Hydrogen Peroxide." Hydrogen Peroxide Natural Traditional Remedies. Web.
[ix] Bond, Allison. "Naturally Produced Hydrogen Peroxide Summons White Blood Cells to
Wounds." Discover. Kalmbach Publishing Co., 04 June 2009. Web.
[x] Lazonby, John. "Hydrogen Peroxide." The Essential Chemical Industry Online. University of
York Centre for Industry Education Collaboration, York, UK, 06 Nov. 2016. Web.
[xi] "Supplier Efforts." Sustainability | P&G. Web.
[xii] "What Is Polyvinyl Alcohol?" Honestly... The Honest Company Blog. 12 June 2014. Web.
[xiii] "Sustainable Manufacturing." Manufacturing | Sustainability - Tide. Web.
[xiv] Brunsman, Barrett J. “Here's How P&G Plans to Make Tide with Wind Power.”
Bizjournals.com, Cincinnati Business Courier, 19 Oct. 2015. Web.
[xv] Abrams, Rachel. “Procter & Gamble to Run Its Factories With Wind Power.” The New York
Times, The New York Times, 19 Oct. 2015.
[xvi] solubletechs 2016. “water soluble film pouch packaging machine for laundry detergent
pods.” Online video clip. Youtube. Youtube, 13 Dec 2016. Web.
[xvii] "About PET." About PET | PETRA: Information on the Use, Benefits & Safety of PET
Plastic. Web
[xviii] Majewski, W. Addy, and Hannu Jääskeläinen. “What Is Diesel Fuel.” DieselNet.com,
Ecopoint, Aug. 2016.
[xix] "Transportation." Sustainability | P&G. Web.
[xx] "Find Out How High Efficiency Washers Work!" Find Out How High Efficiency Washers
Work! - Tide. Web.
[xxi] "Concentrated Detergent." Compaction | Sustainability - Tide. Web.
[xxii] LeBlanc, Rick. "How EcoStar Recycles PET Into Food Grade Packaging." The Balance.
The Balance, 29 Dec 2017. Web.
Bibliography
"About PET." About PET | PETRA: Information on the Use, Benefits & Safety of PET
Plastic. Web
Abrams, Rachel. “Procter & Gamble to Run Its Factories With Wind Power.” The New York
Times, The New York Times, 19 Oct. 2015.
Bond, Allison. "Naturally Produced Hydrogen Peroxide Summons White Blood Cells to
Wounds." Discover. Kalmbach Publishing Co., 04 June 2009. Web.
Brunsman, Barrett J. “Here's How P&G Plans to Make Tide with Wind Power.”
Bizjournals.com, Cincinnati Business Courier, 19 Oct. 2015. Web.
Busch, Sandi. "How Is Citric Acid Made & Where Does It Come From?"
LIVESTRONG.COM. Leaf Group, 03 Oct. 2017. Web.
"Citric Acid: Definition, Safety, Cleaning Uses, & More." The Spruce. Web.
"Concentrated Detergent." Compaction | Sustainability - Tide. Web.
"Find Out How High Efficiency Washers Work!" Find Out How High Efficiency Washers
Work! - Tide. Web.
"Glycerin Suppliers & Producers." P&G Chemicals. Web.
"Hydrogen Peroxide." Hydrogen Peroxide Natural Traditional Remedies. Web.
Ipatenco, Sara. "What Products Contain Citric Acid?" LIVESTRONG.COM. Leaf Group, 03 Oct.
2017. Web.
J, Michael. "How to Make Glycerin From Vegetable Oil." Sciencing. Leaf Group, 03 June
2017. Web.
"KPI of TRIR & DART of Renewable Energy Company | BOP Oversight." EDF Renewable
Energy. Web.
"Laundry Detergent Pacs | Tide PODS®." Laundry Detergent Pacs | Tide PODS® Original. Web.
Lazonby, John. "Hydrogen Peroxide." The Essential Chemical Industry Online. University of
York Centre for Industry Education Collaboration, York, UK, 06 Nov. 2016. Web.
LeBlanc, Rick. "How EcoStar Recycles PET Into Food Grade Packaging." The Balance.
The Balance, 29 Dec 2017. Web.
"New Uses for Crude Glycerin from Biodiesel Production." EXtension. Web.
Majewski, W. Addy, and Hannu Jääskeläinen. “What Is Diesel Fuel.” DieselNet.com,
Ecopoint, Aug. 2016.
P&G Chemicals, “Superol KPO Kosher, USP/FCC/EP,” technical datasheet.
"The Results Are In: Tide Is America's #1 Detergent." America's #1 Laundry Detergent
Tide. Web.
Robinson, Allan. "What Is Glycerin Made From?" LIVESTRONG.COM. Leaf Group, 03 Oct.
2017. Web.
solubletechs 2016. “water soluble film pouch packaging machine for laundry detergent
pods.” Online video clip. Youtube. Youtube, 13 Dec 2016. Web.
"Supplier Efforts." Sustainability | P&G. Web.
"Sustainable Manufacturing." Manufacturing | Sustainability - Tide. Web.
"Texas Wind Farm to Generate 370,000 MWh of Electricity a Year." EDF Renewable Energy. 05
Oct. 2016. Web.
"Transportation." Sustainability | P&G. Web.
"Understanding High Efficiency." Detergents - Tide. Web.
Warmflash, David. “How Are Cellular Respiration & Photosynthesis Almost Opposite
Processes?” Sciencing, Leaf Group Ltd., 24 Apr. 2017
"What Is Polyvinyl Alcohol?" Honestly... The Honest Company Blog. 12 June 2014. Web.
Whitehouse, Jordan. "How to Extract the Citric Acid From Lemons." LEAFtv. Web.
DES 40A Section A01
C.J. Paghasian
INTRODUCTION
When gullible millennials film themselves popping Tide PODS into their mouths, they often do not consider the environmental effects of their actions. Tide POD production creates chemical byproducts, fossil fuels, and plastic debris that could potentially release into waterways and municipalities. Despite these problems, Tide advocates for sustainability across all steps of production. This essay will assess how well Tide adheres to its eco-friendly mission. Tide does its best to minimize the carbon footprint Tide PODS leave behind by working with sustainable companies, reusing materials, and preventing harmful runoff.
RAW MATERIALS ACQUISITION
Tide PODS use many ingredients you wouldn’t normally find in your local farmer’s market. The detergent’s chemical composition is often synthetically manufactured by other companies, which makes most of the ingredients secondary raw materials. Tide does its best to trace how raw materials are acquired, and change or dissociate with companies that do not follow their sustainability parameters.
POLYVINYL ACETATE
Commonly known as PVA, polyvinyl acetate is a clear, dissolve-able film that covers the detergent in a Tide POD. Tide receives its PVA from Monosol, a company which has many facilities across Indiana (Knight). Often, factories that produce PVA release the material into surrounding areas as a gas. The Agency for Toxic Substances and Disease Registry reports that in industrial areas in Houston, gaseous PVA has been measured at 0.5ppm in air, enough to be detected by smell. Usually, though, this dissipates throughout an organism’s body and is released in the form of CO2. PVA, when in the air, has a half-life of about 6 hours; as a liquid, it has a 7-day half-life. Only in incredibly high concentrations does PVA cause throat irritation, coughing, blisters, or skin corrosion (“Public Health Statement for Vinyl Acetate”). No sources indicated how much PVA Monosol releases into the environment, but it’s safe to assume if the company does, PVA is released in harmless amounts.
PALM OIL
All Tide detergents utilize palm oil to make glycerine, enhancing the product’s smoothness. Proctor and Gamble (P&G), the company which processes many of Tide’s chemicals, is one of the largest suppliers of glycerine in the world (“Glycerine”). The palm oil plantations that P&G receive oil from are mostly based in Borneo and Indonesia. Trucks and trains transport palm bundles from the plantations to refineries in Indonesia and Malaysia. The oil is then brought to P&G factories through containers on tanker ships (“Map”). Transporting palm oil across these distances involves a large amount of fossil fuels. The most damaging effect palm oil plantations have on the environment, though, is deforestation. Journalists visiting palm oil plantations in Central Kalimantan, Borneo, saw that “phosphate and other chemicals are polluting the rivers and surroundings of palm plantations,” and that “canals… [had to]... be dug to drain the peat … [and]... dried out peat burns easily, releasing massive amounts of greenhouse gases.” The plantation mainly served Wilmar, a company not associated with Tide (Lang). From the P&G website, the company clearly states that it is strongly opposed to unsustainable deforestation, and “continues work with RSPO, World Wildlife Fund and industry stakeholders to eliminate deforestation in the palm supply chain” (“Sustainability”). Furthermore, if a company does not comply with its strict rules on palm oil acquisition, “P&G will suspend or eliminate palm oil purchases from that supplier.” This is an example of how Tide monitors the environmental effects of the companies from which it receives its ingredients.
ENZYMES
Enzymes are present in all organisms. They acting as catalysts for biological processes, often by breaking down molecules. In detergents, enzymes break down stains. Tide products contain the enzymes protease, amylase, mannanase, and glucanase (McCoy). The use of these proteins decreases Tide’s dependence on petroleum oil, and allow customers to reduce their carbon footprint by “[enabling] washing machines to perform efficiently at 30º C, using much less energy” (“A Non-Scientist’s Guide To Industrial Enzymes”). Enzymes are also biodegradable, since they are made of organic material.
POLYMERS
The main function of polymers in Tide detergents is to keep stains from reattaching onto clothes after being removed. The company BASF produces many of the polymers used in Tide detergents such as polyethylenimine ethoxylate, polyethyleneimine propoxyethoxylate, and glycerine. Their website contains calculated numbers of their waste output, where the company reveals that it has been steadily decreasing its “air pollutants” by thousands of metric tons, and between 2016 and 2015, reduced its heavy metal output by 1 metric ton (“Air and soil”).
MANUFACTURING, PROCESSING, AND FORMULATION
Tide maximizes the reuse and recycling of materials throughout the manufacturing process. P&G owns the factories where Tide’s detergent is formulated, including Tide PODS. Unfortunately, the chefs at Tide keep their detergent-formulating techniques a secret. Their methods must not be different from how most other detergents pods are created. Liquid detergent is typically made by combining solid and liquid ingredients in a “blender” (“Soaps & Detergents: Manufacturing”). Conveyor belts fill up PVA-coated metal pockets - essentially flexible muffin pans - with this liquid (Chen). Tide has been very open about how they recycle the materials, especially their reuse of oil, water, and gasoline.
On the Tide website, the company lists a number of detergent factories that have been working to reduce and reuse materials whenever possible. In Lima, Ohio, the P&G factory reuses all byproducts of the detergent manufacturing process, recycling 1,185 tons of material in 2015, which effectively “[conserved] enough oil to heat and cool approximately 2600 homes for 1 year and enough gasoline to drive more than 763,000 miles.” The manufacturing plant in Alexandria, Louisiana, in a single year has “conserved more than 40 million gallons of water (enough to meet the daily, fresh-water needs of more than 500,000 Americans)” (“Sustainable Manufacturing”).
Factories in India, which seem to be in charge of packaging the detergent as well as chemical processing, have also made very large efforts to reuse and recycle materials. The P&G plant in Baddi has turned 575 tons of scrap metal into material for chairs and cement. A factory in Goa “has recycled and reused over 4000 tons of scrap, which equates to the daily paper & plastic waste generated by 12 times the population of a major Indian metro.” Last year, the Bhiwadi plant has saved 13.3 million liters of water (“Environmental Sustainability Initiatives in India”). P&G also reuses its chemicals by distilling and re-blending them to send to other factories (“Manufacturing Facilities and Operations”).
DISTRIBUTION & TRANSPORT
After manufacturers create the secondary raw materials, large containers in tanker ships bring these chemical ingredients to packaging factories through. After the pods are created and packaged, semi trucks deliver the products to retailers (“Map”). The Toledo Blade reports that about 1,000 semi trucks come through the P&G plant in Lima, Ohio every day (McKinnon). Since 2010, though, P&G has reduced its reliance on trucks by 25% by minimizing “molecular tourism”, or unnecessary transport of materials and products, and using many types of vehicles for transportation (“Transportation”). The Guardian notes that this reduction is due to P&G’s movement from “road to rail,” increasing their use of trains and boats. This change has been “eight times more carbon efficient than relying on trucks” (Beavis). P&G has been actively cutting back on its carbon emissions by altering modes of transportation.
USE, REUSE & MAINTENANCE
Unless dripping down the throat of a shortsighted Youtuber, after use, the contents of a Tide POD are flushed down a washing machine’s drain which feeds into a sewer system. The chemicals that do not adhere to the user’s clothes escape into other water systems, usually lakes, rivers, and oceans. The main concern about this runoff is its potential for eutrophication, which is when extra nutrients from detergents (namely, phosphates) are fed to marine algae, causing algal blooms. Tide has “replaced phosphates in over 95% of [its] cleaning products” prior to 2016 (“Phosphates”). Runoff from Tide detergents mainly consists of enzymes and other organic chemicals, which are biodegradable. Glycerine, one of the main secondary raw materials in detergents made from palm oil, have no effect on the total organic carbon (TOC) of soil (Soerens). According to a study about the bio degradation of surfactants, the chemicals which produce soapiness in Tide PODS, aerobic bacteria decompose surfactants in sewer systems before they get to the ocean. Some areas of runoff, like “river sediments, subsurface soil layer and anaerobic sludge digesters of wastewater treatment plants” require anaerobic decomposition of surfactants by specific organisms (Jelen and Merrettig-Bruns).
RECYCLING
Tide PODS use two types of packaging: a plastic seal-able bag and a plastic container. The bag is made of plastic number one, also known as PET or polyester. One of the most widely used plastics, PET typically used in clear packaging, like water bottles. About 29% of all #1 plastics are recycled (“Common Plastics #1 to #7”). Recycled materials are often sorted by weight by Material Recovery Facilities (MFRs). PET is one of the easiest plastic to recycle. Hundreds of pounds of this plastic is put into bales and shipped off to recycling facilities. Forklifts plop plastic onto conveyor belts, where laser sorts plastic by color. The plastic is then washed so labels come off, and crushed into small pieces. These small pellets are then shipped to U.S., China, and many other places to be reused in carpets, textiles, more bottles, etc. (Winter). Tide has also partnered with Terracycle to recycle all packaging, including the containers made of type 2 plastic. Terracycle recycles the containers in much the same way as the plastic bags. They are crushed into small pellets and sold to other companies for reuse (“Tide® Laundry Bottle Recycling Program”). This is how most of the Tide POD containers are recycled.
WASTE MANAGEMENT
If not properly recycled, Tide POD packaging is thrown into landfills. A Master student’s thesis calculates the rough amount of waste produced from Tide PODS’ packaging bags. Each package creates about 16 g of plastic waste, which equates to 29 lbs of plastic waste per packaging box, or 1,450 lbs package waste per truck (Mikelson). When thrown away, all this plastic material goes to waste. Plastic can take 1,000 years or more to decompose in landfills (LeBlanc). The best way to dispose of Tide POD packaging is to recycle the material.
CONCLUSION
Tide maintains their sustainability mission in Tide PODS by monitoring all aspects of the production process, and minimizing carbon emissions at every step. The detergent is comprised of biodegradable chemicals such as PVA, enzymes, and surfactants. The company is opposed to the massive amounts of deforestation created by the palm oil industry, and works to use palm oil that does not have deleterious effects on the environment. P&G factories that manufacture Tide POD chemicals reuse and recycle chemicals, water, oil, and fuel. Using trains to move products has reduced the carbon footprint of transportation. Tide minimizes the use of ingredients that could cause eutrophication in marine environments. If not thrown into a landfill, the packaging for Tide PODS is easily recyclable. All of this is evidence of Tide’s efforts to make detergent pods that are not only made to deter toddlers and teenagers from eating them, but also with environmental awareness and minimal contribution to climate change.
Bibliography
“A Non-Scientist’s Guide To Industrial Enzymes.” Dupont Industrial Biosciences, n.d., http://biosciences.dupont.com/about-us/guide-to-industrial-enzymes/. Accessed Mar. 3, 2018.
Beavis, Simon. “Procter & Gamble - greening up logistics.” Guardian, 26 May 2011, https://www.theguardian.com/sustainable-business/procter-gamble-greens-logistics. Accessed Mar. 3, 2018.
Chen, Garmen. “Laundry detergent pods packaging machine laundry washing capsule filling machine.” Youtube, uploaded by Garmen Chen, 11 Dec. 2016, https://www.youtube.com/watch?v=7fLzHWyhTaw. Accessed March 3, 2018.
“Common Plastics #1 to #7.” Life Without Plastic, n.d., https://www.lifewithoutplastic.com/store/common_plastics_no_1_to_no_7#.Wql4g5ch3IW. Accessed March 3, 2018.
“Environmental Sustainability Initiatives in India.” P&GIndia.com, n.d., https://www.pg.com/en_IN/sustainability/environmental_sustainability/environmental-sustainability-initiatives-in-india.shtml. Accessed Feb. 30, 2018.
“Glycerine.” P&G Chemicals, n.d., https://www.pgchemicals.com/oleochemicals-products/glycerin.aspx. Accessed March 3, 2018.
Jelens, Erich and Ute Merrettig-Bruns. “Anaerobic Biodegradation of Detergent Surfactants.” Materials, vol. 2009, no. 2, 2009, pp. 181-206.
Knight, Meribah. “The so-secretive company behind Tide Pod detergent packs.” Chicago Business, 7 Aug. 2013, http://www.chicagobusiness.com/article/20131207/ISSUE01/312079982/the-so-secretive-company-behind-tide-pod-detergent-packs. Accessed Mar. 3, 2018.
Lang, Chris. “Guest Post: Central Kalimantan’s oil palm catastrophe in pictures.” REED, 9 Jan. 2013, http://www.redd-monitor.org/2013/01/09/guest-post-central-kalimantans-oil-palm-catastrophe-in-pictures/. Accessed Feb. 30, 2018.
LeBlanc, Rick. “How Long Does It Take for Garbage to Decompose?” Balance, 20 Feb. 2018. https://www.thebalance.com/how-long-does-it-take-garbage-to-decompose-2878033. Accessed March 3, 2018.
“Manufacturing Facilities and Operations.” P&G Chemicals, n.d. https://www.pgchemicals.com/about-pg-chemicals/manufacturing-facilities-and-operations. Accessed Mar. 3, 2018.
“Map.” Tide Laundry Detergent: The Indirect Implication of What We Buy, n.d. http://sites.tufts.edu/sarinatscott/map/. Accessed Feb. 30, 2018
McCoy, Michael. “P&G and Henkel go head to head in the laundry aisle: Makers of Tide and Persil dig deep for ingredients in battle to be the best detergent. Chemical & Engineering News, 23 Jan. 2017, https://cen.acs.org/articles/95/i4/PG-Henkel-head-head-laundry.html?h=1384844903. Accessed Mar. 3, 2018.
McKinnon, Julie. “Big demand flowing in, Tide rolling out in Lima.” Toledo Blade, 5 Jun. 2005, http://www.toledoblade.com/Economy/2005/07/05/Big-demand-flowing-in-Tide-rolling-out-in-Lima.html. Accessed Mar. 3, 2018.
Mickelson, Aaron. “Solution 1: Tide PODS.” The Disappearing Package, n.d. https://disappearingpackage.com/solutions/tide/. Accessed Feb. 30, 2018.
“Phosphates.” P&G, n.d., https://us.pg.com/our-brands/product-safety/ingredient-safety/phosphates. Accessed Mar. 3, 2018.
“Public Health Statement for Vinyl Acetate.” Agency for Toxic Substances & Disease Registry, 21 Jan. 2015, https://www.atsdr.cdc.gov/PHS/PHS.asp?id=669&tid=124. Accessed Feb. 25, 2018.
“Soaps & Detergents: Manufacturing.” American Cleaning Institute, n.d., http://www.cleaninginstitute.org/clean_living/soaps__detergents_manufacturing.aspx. Accessed Mar. 3, 2018.
Soerens, Thomas. “Biodiesel Waste Products as Soil Amendments - Toxicity, Runoff, and Growth.” American Water Resources Association, 27 Mar. 2013, http://www.awra.org/meetings/Spring2013/doc/PP/Sess%2043%20abs.pdf. Accessed Mar. 3, 2018.
“Sustainability.” P&G Chemicals, n.d., https://www.pgchemicals.com/sustainability. Accessed Mar. 3, 2018.
“Sustainable Manufacturing.” Tide, n.d. https://tide.com/en-us/about-tide/sustainability/manufacturing. Accessed Feb. 30, 2018.
“Tide® Laundry Bottle Recycling Program.” Terracycle, n.d., https://www.terracycle.com/en-US/brigades/tide. Accessed March 3, 2018.
“Transportation.” P&G, n.d., https://us.pg.com/sustainability/environmental-sustainability/focused-on/transportation. Accessed Mar. 3, 2018.
Winter, Debra. “The Violent Afterlife of a Recycled Plastic Bottle.” Atlantic, 4 Dec. 2015, https://www.theatlantic.com/technology/archive/2015/12/what-actually-happens-to-a-recycled-plastic-bottle/418326/. Accessed March 3, 2018.