Design Life-Cycle
assess.design.(don't)consume
Kelvin Wu
Yoselin Diaz, Sierra Goodfriend
DES 40A
Professor Cogdell
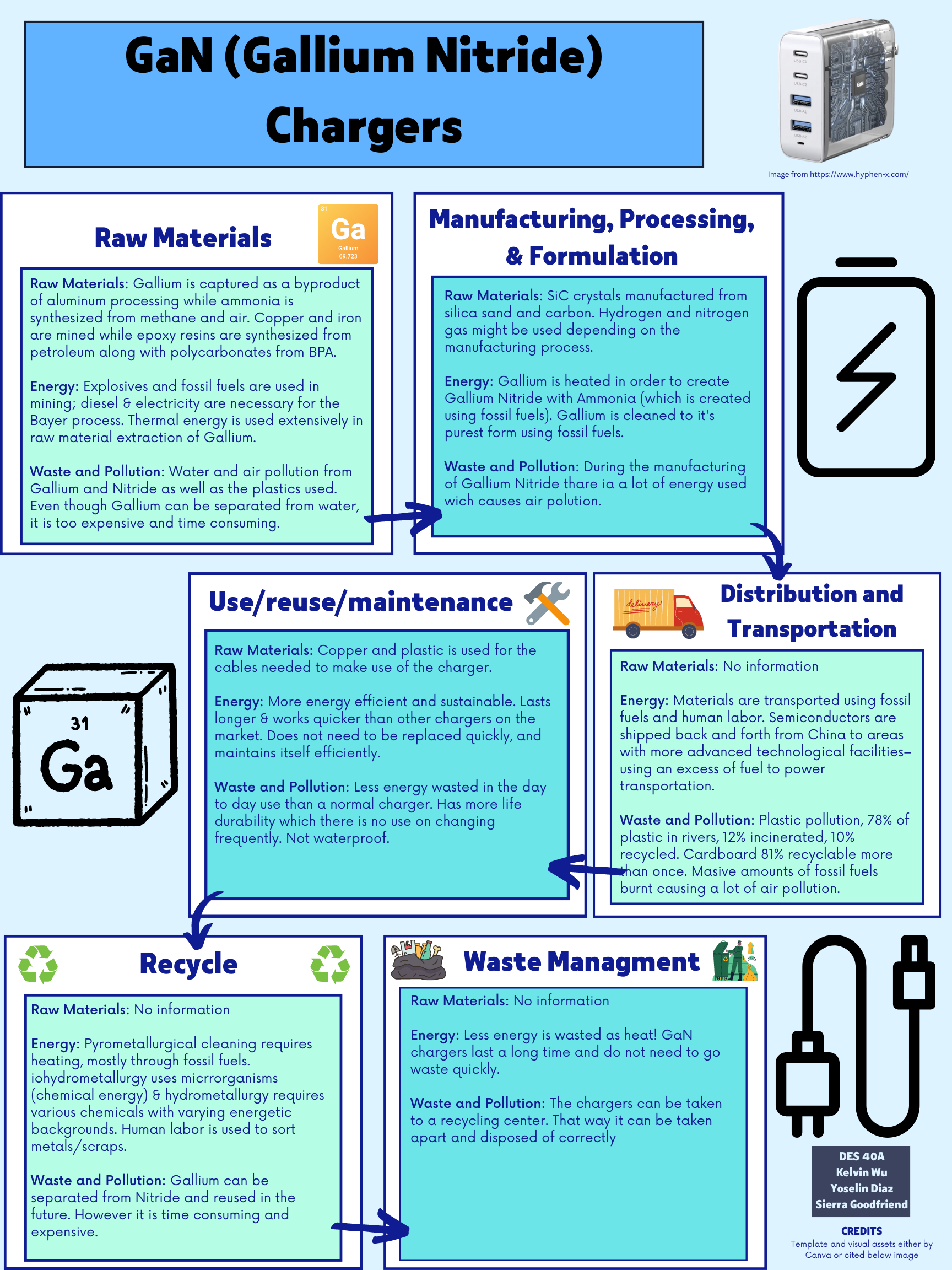
Materials in GaN Chargers
Gallium nitride is a material replacing the familiar silicon in the power adapters, or chargers, that have become universal in our lives due to the rise of smartphones and handheld electronics. Since the beginning, these adapters have been made with silicon as the primary semiconductor material for transistors used inside their integrated circuits. However, silicon has its limits, dictated by its physical properties. Thus, the search for a new material has led to gallium nitride, or GaN, as a replacement for silicon within our power adapters. This change in material alone shrinks the size of the adapters, allowing for powerful laptop chargers to be fitted in the size of silicon-based phone chargers. GaN-based power adapters are also more efficient in their power processing, leading to energy savings over the long run. While GaN chargers are not able to solve the problem of plastics usage, the switch to gallium nitride takes advantage of abundant waste materials repurposed to be used as a powerful successor to silicon without large scale environmental impacts from material acquisition compared to its silicon counterpart.
The defining characteristic for GaN chargers is, of course, their use of gallium nitride as the semiconductor material. Gallium, in its raw form, is primarily produced as a byproduct in the refining process of bauxite ore, the main source of aluminum (U.S. Geological Survey 73). Bauxite ore is mined directly from the ground and refined using the Bayer Process, during which gallium is separated into and subsequently extracted from the process liquids. This separation is done via ion exchange using a resin called Duolite ES-346 (Zhao et al.). Reliance on aluminum production is actually a positive factor when considering the availability of raw materials, as aluminum is such a widely used material that relying on the Bayer process for gallium extraction is expected to feed the world’s gallium needs for the foreseeable future (Frenzel et al.). Because of this, it could be argued that raw gallium production has practically no impact on the environment, as it is merely a byproduct of a process already being performed. Although gallium is incredibly abundant and available, nearly all of the world’s gallium supplies come from China (“World Mineral Statistics Data”), thus resource security cannot be guaranteed on a global scale as there would be no alternative providers for gallium in quantities of this scale if China were to stop exporting. While current gallium supplies largely depend on the aluminum refinement process (about 90%), there are still other sources that we can turn to if needed. Gallium can also be extracted from refining sulfidic zinc ore, albeit not as efficiently as bauxite (Frenzel et al.). Even though China is also the largest producer of zinc ore, they are not the sole supplier the way they are with gallium as Peru and Australia also produce substantial quantities of zinc (U.S. Geological Survey 201), which rivals China’s production when combined. Besides zinc, coal ash is yet another possible source of gallium, with an estimated potential about 1.3 times the world’s current gallium production (Frenzel et al.), although no gallium is currently being produced this way. Thus, there is little reason to believe that gallium will become a scarce resource for the time being. While it may not be renewable, it is so abundant that availability does not need to be a factor of concern.
After gallium, the other major component in gallium nitride is nitride. The main source of nitrogen in the manufacturing process currently is ammonia. Ammonia is most commonly synthesized using the Haber-Bosch process. This process requires a source of hydrogen, primarily purified from steam and methane, and nitrogen, generally sourced straight out of the atmosphere (“Introduction to Ammonia Production”). Similar to gallium, ammonia also has no issues with scarcity as a resource, due to the sheer abundance of its materials, which explains why it is the main fertilizers used in crop production all around the world.
Gallium nitride is typically manufactured in two different ways, either through metalorganic chemical vapor deposition (MOCVD) or through molecular beam epitaxy (MBE). Both of these processes use pure gallium as the base material and ammonia as a source of nitrogen (Chen 20). However, due to the expense and difficulty to grow gallium nitride crystals on top of themselves while keeping quality, and therefore usability, high, silicon carbide (SiC) is most often used as a substitute material for the base which GaN crystals can then grow on (Lidow 11). SiC crystals are manufactured from silica sand, commonly mined, and carbon in the form of coke, coal baked to maximize its carbon content (Dhanaraj et al.). Sapphire crystals and pure silicon crystals can also be used as a base, but the resulting GaN crystals from either of these substitutes don’t perform as well in applications involving thermal conductivity, such as power adapters (Lidow 11). MOCVD additionally requires either pressurized hydrogen or pressurized nitrogen as a carrier gas in order to help facilitate the transport and growth of gallium crystals (Chen 26). MBE, on the other hand, does not require a carrier gas, but cannot grow crystals as quickly as MOCVD (Medjdoub and Iniewski). Hence, MOCVD is currently the main production method of GaN crystals; its efficiencies are worth the use of carrier gasses, which we have a plentiful supply of.
Besides the gallium nitride crystals themselves, GaN chargers also contain much of the same internals as regular silicon chargers. Loops of copper cable are wrapped around a magnetic iron core to lower the voltage of mains power (“How Does an AC Adaptor Work?”). Copper as a raw material is mined and smelted in order to remove impurities. Additives such as silica and limestone can be added while smelting to further purify the copper (“Manufacturing Process of Copper”). Iron is very similar in that it is also mined, but it must be crushed into lumps along with clay before being smelted (“How Iron is Produced”). Neither of these two metals are at risk of running out due to their abundance in the Earth’s crust (U.S. Geological Survey 63, 99). The circuit board inside the charger also makes use of copper, along with epoxy resin and glass fiber (“Complete Introduction to Copper Clad Laminate”). Epoxy resin is extracted from petroleum, which creates concern as oil drilling can cause great environmental impacts, such as water contamination and oil spills (“Oil and the Environment”). Not only does petroleum have a huge impact on the environment, but it is also not a renewable resource. Due to the fact that we rely on fossil fuels for 80% of our energy (“Nonrenewable Resources”), the crude oil needed for epoxy resin is at risk of becoming a scarce resource. Copper and epoxy resin also see use as heat spreaders in chargers (J. Weimer et al.), thus further increasing the use of materials manufactured from nonrenewable resources. Lastly, while dependent on the manufacturer of the charger, the outer shell will most likely be made of plastic, usually polycarbonates. Polycarbonates are manufactured from bisphenol A (BPA) and phosgene (“Polycarbonate (PC)”). BPA is known for leeching into the human body, although this is mostly done through food packaging (“Bisphenol A (BPA)”). However, these materials are standard when it comes to charging and even electrical products in general, so while the search for more environmental materials is much needed, this is not an issue isolated to GaN chargers.
Once bought, GaN chargers must be paired with a charging cable in order to be used. Again, cables may differ slightly depending on what brand they are, but they are mostly copper cores with a layer of plastic insulation wrapped around them (“Cable Materials”). As mentioned above, copper is currently an abundant resource. Therefore, strengthened by the fact that most cables are reused until they break, the raw materials involved in the production of power cables for the use of GaN chargers is not significant.
GaN chargers bring a new level of charging to our battery-filled lives. They shrink charger sizes, create more powerful chargers, and do it all more efficiently all with the change of a single material: swapping silicon for gallium nitride. These benefits could help lower environmental impacts from e-waste by allowing consumers to buy less. The increase in power means consumers can consolidate multiple chargers down to one, reducing the amount of plastics used in the creation of outer casings. Since these benefits don’t come with any drawbacks compared to silicon chargers, they leave GaN as the next step in the continuous evolution of consumer electronics. Gallium is not only ridiculously abundant, but a large majority of the world’s supply is also generated as a byproduct of another material. Ammonia production has also been engineered to be very efficient due to its use as a fertilizer, making it cheap and plentiful. With this in mind, there is no reason not to switch to gallium nitride as the new material for the semiconductors in our chargers.
Works Cited
“A Complete Introduction To Copper Clad Laminate (CCL) (2022) - JHYPCB.” PCB Prototype Manufacturing & Assembly Services - JHYPCB, https://www.pcbelec.com/pcb-materials/copper-clad-laminate-2. Accessed 16 Mar. 2023.
Bi, Wengang (Wayne), et al., editors. Handbook of GaN Semiconductor Materials and Devices. 1st ed., CRC Press, 2017. DOI.org (Crossref), https://doi.org/10.1201/9781315152011.
“Bisphenol A (BPA).” National Institute of Environmental Health Sciences, https://www.niehs.nih.gov/health/topics/agents/sya-bpa/index.cfm. Accessed 16 Mar. 2023.
“Cable.” Encyclopedia Britannica, https://www.britannica.com/technology/cable-electronics. Accessed 16 Mar. 2023.
“Cable Materials | Metal Types Used in Cables & Wire | Cable Conductivity.” PIC Wire & Cable, https://www.picwire.com/resources/technical-articles/electronic-cable-materials/. Accessed 16 Mar. 2023.
Chen, Jr-Tai. MOCVD Growth of GaN-Based High Electron Mobility Transistor Structures. 1st ed., vol. 1662, Linkopings Universitet, 2015, https://ebookcentral.proquest.com/lib/ucdavis/detail.action?docID=3328196.
Dhanaraj, Govindhan, et al. “Growth and Characterization of Silicon Carbide Crystals.” Springer Handbook of Crystal Growth, edited by Govindhan Dhanaraj et al., Springer Berlin Heidelberg, 2010, pp. 797–820, https://doi.org/10.1007/978-3-540-74761-1_23.
Frenzel, Max, et al. “On the Current and Future Availability of Gallium.” Resources Policy, vol. 47, Mar. 2016, pp. 38–50. DOI.org (Crossref), https://doi.org/10.1016/j.resourpol.2015.11.005.
GaN Power ICs | Navitas. https://navitassemi.com/gan-power-ics/. Accessed 15 Mar. 2023.
“How Does an AC Adaptor Work?” Techwalla, https://www.techwalla.com/articles/how-does-an-ac-adaptor-work. Accessed 16 Mar. 2023.
“How Iron Is Produced.” Metal Casting Institute, 5 Apr. 2020, https://metalcastinginstitute.com/how-iron-is-produced/.
Introduction to Ammonia Production. 8 Sept. 2016, https://www.aiche.org/resources/publications/cep/2016/september/introduction-ammonia-production.
J. Weimer, et al. “Miniaturization and Thermal Design of a 170 W AC/DC Battery Charger Utilizing GaN Power Devices.” IEEE Open Journal of Power Electronics, vol. 3, 2022, pp. 13–25, https://doi.org/10.1109/OJPEL.2021.3137093.
JD. “What Is Epoxy Resin and How Does It Work.” Resin Affairs, 27 June 2021, https://resinaffairs.com/what-is-epoxy-resin/.
Lidow, Alex. GaN Transistors for Efficient Power Conversion. Second edition, Wiley, 2014.
“Manufacturing Process of Copper.” ThoughtCo, https://www.thoughtco.com/copper-production-2340114. Accessed 16 Mar. 2023.
Medjdoub, Farid, and Krzysztof Iniewski, editors. Gallium Nitride (GaN): Physics, Devices, and Technology. CRC Press, 2016.
National Center for Biotechnology Information. Hazardous Substances Data Bank (HSDB) : 162. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/162#section=Methods-of-Manufacturing-(Complete). Accessed 15 Mar. 2023.
Nonrenewable Resources. https://education.nationalgeographic.org/resource/nonrenewable-resources. Accessed 16 Mar. 2023.
Oil and the Environment - U.S. Energy Information Administration (EIA). https://www.eia.gov/energyexplained/oil-and-petroleum-products/oil-and-the-environment.php. Accessed 16 Mar. 2023.
Polycarbonate (PC) - Properties, Uses, & Structure - Guide. https://omnexus.specialchem.com/selection-guide/polycarbonate-pc-plastic. Accessed 16 Mar. 2023.
PubChem. Methane. https://pubchem.ncbi.nlm.nih.gov/compound/297. Accessed 16 Mar. 2023.
Rogers, Kara. “Bisphenol A.” Encyclopedia Britannica, https://www.britannica.com/science/bisphenol-A. Accessed 15 Mar. 2023.
Swain, Basudev, et al. “Recycling of GaN, a Refractory EWaste Material: Understanding the Chemical Thermodynamics.” International Journal of Applied Ceramic Technology, vol. 13, no. 2, Mar. 2016, pp. 280–88. DOI.org (Crossref), https://doi.org/10.1111/ijac.12473.
U.S. Geological Survey. Mineral Commodity Summaries 2023. Report, 2023, 2023, p. 210, https://doi.org/10.3133/mcs2023. USGS Publications Warehouse.
What Are Polycarbonates? Everything You Need to Know | Acme Plastics. https://www.acmeplastics.com/what-are-polycarbonates. Accessed 15 Mar. 2023.
World Mineral Statistics Data | Statistics & Commodities | MineralsUK. https://www2.bgs.ac.uk/mineralsUK/statistics/wms.cfc?method=listResults&dataType=Production&commodity=737&dateFrom=2010&dateTo=2020&country=&agreeToTsAndCs=agreed. Accessed 16 Mar. 2023.
Zhao, Zhuo, et al. “Recovery of Gallium from Bayer Liquor: A Review.” Hydrometallurgy, vol. 125–126, Aug. 2012, pp. 115–24. DOI.org (Crossref), https://doi.org/10.1016/j.hydromet.2012.06.002.
Sierra Goodfriend
Professor Cogdell
DES 040A
16 March 2023
Gallium Nitride Chargers: Life Cycle Energy
Introduction
Technology is always growing, advancing, and changing the way our systems work on local and global scales. Gallium Nitride (GaN) chargers are a relatively new technology that have the potential to change and reshape our energy systems. These chargers utilize energy efficient Gallium Nitride semiconductors that are wide band gap materials, which essentially means that they can “operate at higher voltages, temperatures, and frequencies” (Infineon). These semiconductors allow the charger to save energy and function at an impressive level of efficiency. GaN chargers save energy while being used by consumers, but they also require large amounts of energy in the process of producing the semiconductors and materials within them. Throughout the lifecycle of a GaN charger, raw materials are processed into the chargers before being transported and utilized by consumers. This paper explores the fuel sources and energy used in this process from source to waste with a focus on Gallium Nitride; the material that sets GaN chargers apart from other chargers on the market.
Mining and Cleaning Gallium
Gallium needs to be sourced in order to manufacture Gallium Nitride semiconductors and create effective GaN chargers. While it can be recycled and repurposed, Gallium is often mined up as a raw material. Over 90% of the world’s Gallium is sourced in China through the process of mining and smelting bauxite for aluminum (Cao). Gallium is harvested from bauxite ore as a byproduct. Mining up bauxite requires electricity and fuel–usually diesel. Electricity in China is sourced mostly from coal and natural gas (Statista Research Department). Bulldozers and explosives are used to clear away surrounding land and obtain bauxite ore (GKB), requiring heavy machinery that is run by fossil fuels, as well as human labor. Once bauxite ore has been acquired, Gallium must be smelted out of it. Smelting of Gallium is done using the Bayer process at high temperatures, consuming an abundance of energy–theoretically, 7 GJ/t – 20 GJ/t (Scarsella). It is an energy intensive process that is powered by “fossil fuels such as diesel, gasoline, natural gas and coal,…electricity” (ICSOBA), and human/machine labor (Institut Für Seltene). Despite large amounts of energy being used, a relatively small amount of Gallium is actually sourced in this process. Within bauxite, Gallium is present in small quantities–about 50 parts per million (USGS). In fact, one ton of bauxite only contains about 2.75 ounces of Gallium (AGS). However, as previously mentioned, the Bayer process’s purpose is to acquire aluminum, and Gallium is simply a byproduct. Thus, an argument could be made that energy usage is not intensive in the acquisition of Gallium in the Bayer process as this process will occur whether Gallium is being sourced or not. However, Gallium needs additional processing once it has been acquired. It must be cleaned so that it is in its purest form if it is going to be used in semiconductors. Common cleaning methods are “vacuum distillation, fractional crystallization [and] zone melting” (Institut Für Seltene). Fractional crystallization is the most efficient of these methods as “the overall yield of purification by crystallization is close to 100%” (Cao). However, all three methods require thermal energy and are most likely going to be powered by fossil fuels.
Manufacturing Gallium Nitride
Once Gallium is cleaned and in its purest form, it can be turned into Gallium Nitride and implemented into a semiconductor to be used within a GaN charger. Gallium is often converted to Gallium Nitride by injecting it with ammonia. It is important to note that ammonia itself has a life cycle that is energy intensive as it is created using “fossil fuel–derived hydrogen” (American Chemistry Society). Essentially, in this process, Gallium is heated up and put under pressure, and ammonia is synthetically added to it so that these substances can chemically react and combine (Hal). The temperature range at which Gallium Nitride is produced using ammonia is around 750-1100 degrees celsius–a temperature maintained by fossil fuels (Hal). Using GaN chargers is a more efficient and sustainable option than alternative chargers (due to their high level of efficiency), but the GaN semiconductors within the chargers are sourced by using fossil fuels and energy intensive practices. Once Gallium Nitride is created, it can be implemented into a semiconductor. Most semiconductors are produced in China, although information is not available detailing exactly what factories produce GaN semiconductors and chargers, as well as where they are shipped from. Semiconductor technology has not yet been perfected in China, and so China “relies on importing high-end gallium products (i.e., integrated circuits) from abroad, whose raw materials are originally sourced from China” (Cao). Such a process uses an excess of fossil fuels and natural gas in order to ship materials back and forth. Energy is thus wasted in this process, which calls attention to the necessary advancement of semiconductor technology so that countries can source this technology on their own.
Energy Per User
GaN chargers make a substantial contribution to reducing energy usage after they have been produced. While operating, GaN chargers lose less energy as heat (RAVPower), and thus they are more energy efficient on the level of individual usage. GaN chargers are smaller than other chargers on the market (RAVPower), which ultimately leads to less waste overall. Bulkier chargers require more materials that often end up being thrown away at the end of a charger’s lifecycle. GaN chargers have a “longer lifespan than traditional chargers, making them a more durable and less wasteful (Beck)”, and so every consumer who owns a GaN charger saves energy because their chargers will not have to be replaced as quickly as alternative chargers would. However, it could be argued that because GaN chargers are more expensive, they are not accessible to the masses and therefore are only saving energy in the hands of those who can afford them. Even so, if GaN chargers (and GaN technology at large) are able to replace Silicon based semiconductors in charging devices, energy will be saved on a massive scale. The individual impacts of using and supporting GaN technology can have beneficial impacts on lessening the amount of energy we consume and waste through our electronics.
Recycling Methods and Waste
GaN charger parts are recyclable, but for the most part they go into the landfill because recycling methods are not widespread or perfected. The current recycling rate of Gallium products as a whole is a mere 1% (Cao), and therefore the amount of recycled GaN chargers is negligible compared to the amount thrown away. GaN chargers are constantly being produced and sourced from raw materials, so working on creating better recycling technologies and facilities for GaN chargers is crucial. Fortunately, there are technologies that can be utilized in order to recycle Gallium as e-waste–like pyrometallurgical processing, hydrometallurgical processing and biohydrometallurgy (Dutta) These methods have efficiency rates of roughly 90% (Dutta). Pyrometallurgical processing is achieved through heating metals in smelters at high temperatures, usually using coal and natural gas (Dutta). Hydrometallurgical processing uses acidic and basic chemicals in order to extract metals from products (Dutta). Hydrometallurgical processing can be energy inefficient because the chemicals used in this process are not always sourced in green/energy efficient ways. Finally, biohydrometallurgical processing involves allowing an interaction between microorganisms and metals wherein the microorganisms help to extract the metal in its base form (Dutta). Biohydrometallurgy is a more energy efficient way to retrieve metals and recycle Gallium in GaN chargers because it relies on organisms instead of nonrenewable fuels. Thus, biohydrometallurgy is a preferred way to recycle Gallium in GaN chargers. However, none of these methods are in widespread use as of now. While the potential for efficient e-waste recycling is promising, recycling facilities and recycling systems for Gallium are not efficient enough when compared to the rate of demand for Gallium in our markets. GaN chargers are therefore not recycled to an efficient extent, and energy is wasted in extracting and processing raw materials for these chargers. Even though GaN chargers (and the Gallium within them) end up in landfills rather than being recycled, they last a much longer time than other chargers and are more efficient when actually charging.
Fortunately, recycled materials are being used to an increasing extent that increase energy efficiency in GaN charges. For example, Belkin, a company that sells GaN chargers, has reported that it is using “post consumer recycled plastics (PCR)” (Voloschuk) for the body of GaN chargers. These plastics [reduce] energy consumption by at least 79%” (The US Plastics Pact) and thus help to make Belkin’s GaN chargers more energy efficient. Apple has recently begun manufacturing GaN chargers, and they have reported using only recycled gold in circuit boards (Apple). While recycling gold can be energy intensive, it is usually more efficient than mining gold as a raw material. These are a few of the many examples of recycled materials going into GaN chargers as our need for energy efficiency is what is driving some of the manufacturers of these chargers.
Conclusion
The energy used in the process of extracting materials and manufacturing GaN chargers is far greater than the energy they use while actually charging devices. However, even though most GaN chargers have energy intensive origins, using them is a more efficient and sustainable option than using alternative chargers on the market. GaN is not always accessible or utilized because it is a new technology, and it is fairly expensive in comparison to other chargers. Even so, it is a technology that might aid us in achieving a better level of energy efficiency in our electronic systems in the future. GaN chargers have a life cycle that starts off energy intensive and inefficient, but they are sustainable in their function. They could be manufactured in more sustainable ways, and recycling methods are constantly being worked on. GaN chargers are a promising technology that should not be overlooked by consumers, as they can hopefully one day change our global energy usage for the better.
Works Cited
Alqarqaz, Qusi. “Gallium Nitride: The Ideal Semiconductor for Power-Hungry Electronics.”
IEEE Spectrum, IEEE Spectrum, 24 June 2021,
https://spectrum.ieee.org/gallium-nitride-the-ideal-semiconductor-for-powerhungry-elect
onics.
“Ammonia.” American Chemical Society , 8 Feb. 2021,
https://www.acs.org/molecule-of-the-week/archive/a/ammonia.html.
Apple. “IPhone 14 Pro Max.” Product Environmental Report, Apple, 7 Sept. 2022,
https://www.apple.com/lae/environment/pdf/products/iphone/iPhone_14_Pro_Max_PE
Sept2022.pdf.
Beck, Travis. “Understanding GaN Chargers: The Future of Charging Technology.” Paracable, 2
Mar. 2023,
https://paracable.com/blogs/articles/understanding-gan-chargers-the-future-of-charging-t
hnology.
Çelikel, Bekir, et al. “Energy Consumption Optimization in Alumina Production.” 34th
Conference and Exhibition, ICSOBA, 3 Oct. 2016, https://icsoba.org/proceedings/34th-conference-and-exhibition-icsoba-2016/?doc=20.
Communications and Publishing. “USGS Updates Mineral Database with Gallium Deposits in
the United States: U.S. Geological Survey.” USGS Updates Mineral Database with Gallium Deposits in the United States | U.S. Geological Survey, 11 Mar. 2021, https://www.usgs.gov/news/technical-announcement/usgs-updates-mineral-database-gallium-deposits-united-states.
Deblina Dutta, Rahul Rautela, Lohit Kumar Srinivas Gujjala, Debajyoti Kundu, Pooja Sharma,
Mamta Tembhare, Sunil Kumar, A review on recovery processes of metals from E-waste: A green perspective, Science of The Total Environment, 2023, https://www.sciencedirect.com/science/article/pii/S0048969722074939
“Gallium Price, Occurrence, Extraction, Use: Institute for Rare Earths and Metals.” Institut Für
Seltene, https://en.institut-seltene-erden.de/seltene-erden-und-metalle/strategische-metalle-2/Gallium/.
Fanghai Lu, Tangfu Xiao, Jian Lin, Anjing Li, Qiong Long, Fang Huang, Lihua Xiao, Xiang Li,
Jiawei Wang, Qingxiang Xiao, Haiyan Chen, Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure, Hydrometallurgy, Volume 175, 2018, https://www.sciencedirect.com/science/article/pii/S0304386X17304942
Gallium, Arkansas Geological Survey, https://www.geology.arkansas.gov/minerals/metallic/gallium.html.
General Kinematics, GKB. “Aluminum Mining and Processing: Everything You Need to Know.” General Kinematics, 1 Aug. 2022, https://www.generalkinematics.com/blog/aluminum-mining-processing-everything-need-know/.
Hossein Rabiee Golgir, Yang Gao, Yun Shen Zhou, Lisha Fan, Premkumar Thirugnanam, et al..
LowTemperature Growth of Crystalline Gallium Nitride Films Using Vibrational Excitation of Ammonia Molecules in Laser-Assisted Metalorganic Chemical Vapor Deposition. Crystal Growth & Design, 2014, 14 (12), pp.6248-6253. ff10.1021/cg500862bff. ffhal-03704084f.
Infineon Technologies. “Wide Bandgap Semiconductors (Sic/Gan) - Infineon
Technologies.” Semiconductor & System Solutions - Infineon Technologies, https://www.infineon.com/cms/en/product/technology/wide-bandgap-semiconductors-sic-gan/.
Jaghory, Dillon. “China Sector Analysis: Energy.” Global X, 22 Feb. 2022, https://www.globalxetfs.com/china-sector-analysis-energy.
Scarsella, A.A., Noack, S., Gasafi, E., Klett, C., Koschnick, A. (2015). Energy in Alumina
Refining: Setting New Limits. In: Hyland, M. (eds) Light Metals 2015. Springer, Cham. https://doi.org/10.1007/978-3-319-48248-4_24
Statista Research Department. “China: Electricity Generation Share by Source 2021.” Statista,
Ember, 25 Jan. 2023, https://www.statista.com/statistics/1235176/china-distribution-of-electricity-production-by-source.
Team RAVPower. “What Is a Gan Charger, and Why Do You Need One?” RAVPower, 26 Aug.
2022,
https://blog.ravpower.com/2022/08/what-is-a-gan-charger-and-why-do-you-need-one/.
United States, Congress, Mineral Resources Program, et al. Gallium: A Smart Metal, U.S. Dept.
of the Interior, U.S. Geological Survey, 2013.
United States, Congress, Waldrip, Karen Elizabeth. Molten Salt-Based Growth of Bulk GaN and
InN for Substrates., Sandia National Laboratories. https://www.osti.gov/biblio/920124.
Voloschuk, Chris. “Belkin Mobile Power Products Transition to Recycled Plastics, Plastic ...”
Recycling Today, 24 Jan. 2023,
https://www.recyclingtoday.com/news/belkin-mobile-power-products-to-use-recycled-pla
stics-plastic-free-packaging/.
“Why Use PCR?” The U.S. Plastics Pact, 8 Mar. 2023, https://usplasticspact.org/why-use-pcr.