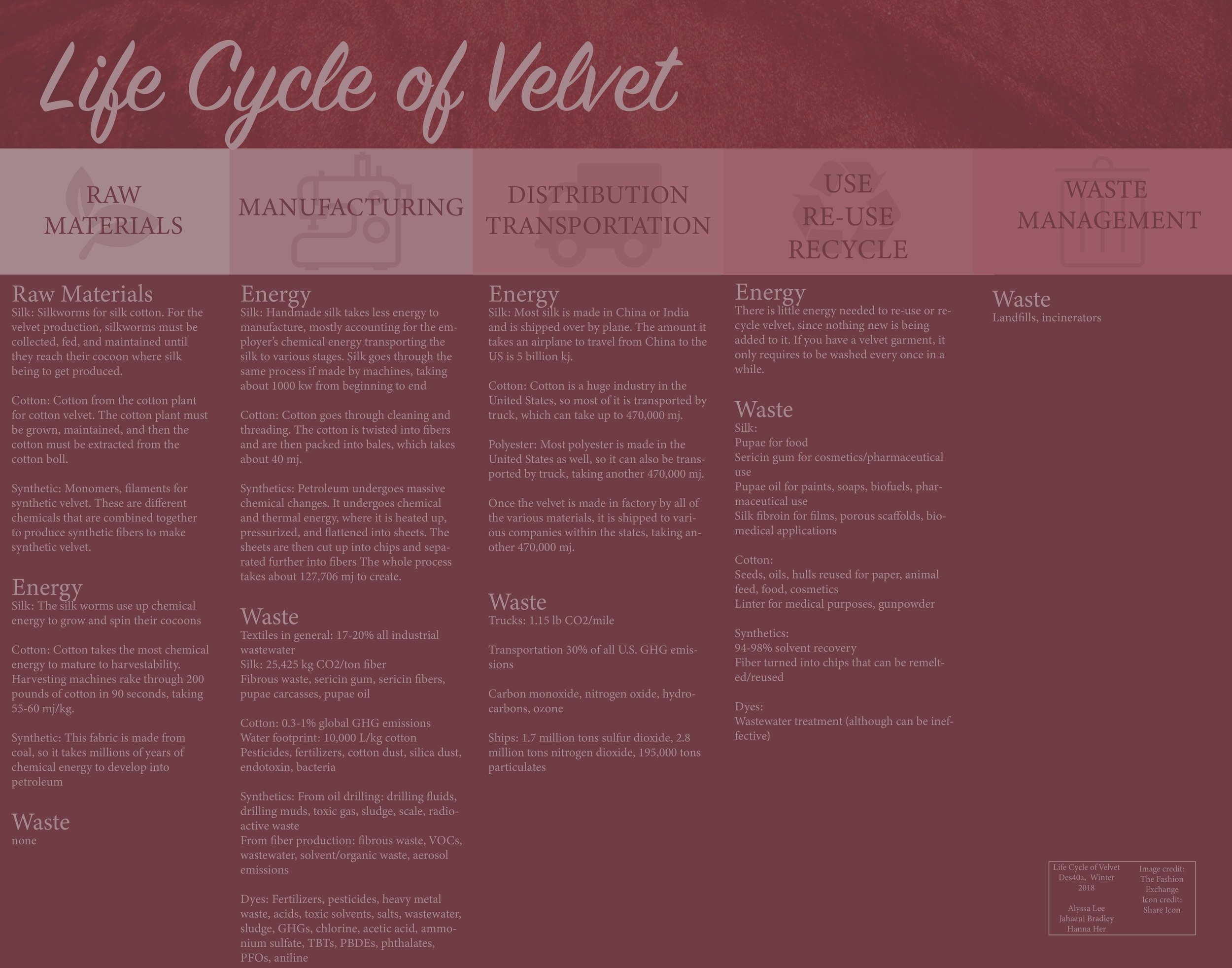
Design Life-Cycle
assess.design.(don't)consume
Velvet Life Cycle
Alyssa Lee
Prof. Cogdell
Des40a
16 March 2018
Embodied Energy in the Making of Velvet
Velvet is a material synonymous with royalty and status, and evidence of it can be traced back to 221 BCE in China. Part of what makes velvet so interesting is that it’s not the material that makes the velvet, it’s how it’s put together. Velvet can be made out of almost any material, ranging from cotton to synthetics and gets its silky texture from the way the fabric is looped and raised, called piles.[1] In ancient times, making velvet was a long and laborious process, made with silk and spun and woven all done by hand. It was quickly discovered by surrounding countries and became a staple on the Silk Road, and became other countries tried to emulate it. Today, velvet is still a sign of luxury but is industrially and quickly made with machines and is made out of a variety of materials to achieve different feels and qualities. The production value and time of making velvet has increased since it was first made, but so has the amount of energy it takes to produce it. The production of velvet involves a large amount of energy, and depending on the type of velvet, can use up to five times the amount of energy. While high amounts of energy can’t exactly be described as efficient, there are vast differences in the efficiencies of the different types of velvet.
Cotton
One of the more common materials to make velvet with is cotton. Cotton is a cheaper material that’s widely and locally produced, being a major industry in the United States, making it easier to obtain than materials like silk. The embodied energy required with the production of velvet starts here. Cotton plants are the longest growing crop in the United States, which means the plant itself takes more chemical energy than other crops just to grow. [2] When the crops are ready to be harvested, farmers use machines to pick the cotton and comb through it, harvesting 200 pounds of cotton in 90 seconds, much more efficiently than if they were to do it by hand.[3] It takes about 55-60 mj/kg to harvest the fiber, which includes the kinetic and electric power used by the machines to pick the cotton and twist it from the cotton heads, separate, and comb through the cotton. The machines also twist the cotton into fibers, which are then packed bales and readied for distribution to textile mills.[4] They are transported by truck, which takes a minimum amount of 114 calories, or 470,000 mj.[5] In the factory, it undergoes a process to spin it into threads and is then transformed by machine into the velvet fabric, taking about 40 mj including dyeing.[6] From there it is distributed across the United States, meaning that overall it takes 470,100 mj to make velvet from cotton.
Synthetics
Velvet can also be made out of synthetic material. The process and energy involved in creating nylon or polyester are extensive starting at the gathering and creation of fossil fuels. The fossil fuels first undergo millions of years of chemical energy to transform organic matter into petroleum. It is then transported to a manufacturing factory where it undergoes a great deal of thermal and chemical energy. The petroleum is heated up and pressurized, which rearranges the chemical structure to form flat sheets of nylon. Kinetic and electric energy is then applied, using machines to cut up the sheets into chips and, separated into fibers, and spun into threads.[7] It takes about 250 mj to just make the chemical, and another 470,000 mj for transportation of the raw oil and transportation of the fibers to a textile mill.[8] The process of creating synthetic fibers uses a massive amount of chemical energy, since they are created by rearranging the chemical structure of oil. The process includes cracking, which breaks the chains of hydrocarbons and rearranges them into many different types of chemicals until it reaches the desired state of polyester or nylon, both of which are used to make synthetic velvet.[9] Even the process after creating the material involves a major amount of energy, as the plastic has to be heated up, measured, filtered, pumped, cooled, and straightened to create a single fiber.[10] In fact, it takes 127,706 mj to make one ton of fabric, taking five times as much energy as it takes to make cotton.[11][12]
Silk
The last and original textile used to make velvet is silk. Part of the reason velvet is revered as a symbol of wealth and importance was because it was laboriously made from silk, which was a luxury fabric in and of itself. Silk can be machine made or handmade, but the process starts with chemical energy in the silkworms and mulberry leaves that they consume. Once they reach maturity, they spin a cocoon made of silk which is then harvested and put into boiling water (about 1.7 kj of thermal energy). As the cocoon unravels into strands, they are removed from the water and set out to be cleaned and dried. The strands can be twisted into threads of silk, which can then be dyed and woven into fabric.[13] Done by hand, silk is much higher in quality, more sustainable, and also uses less energy, as most of it is the energy of the people moving the silk from stage to stage.[14] Done by machine, silk production is more efficient, however one machine takes about 1000 kw from beginning to end. The majority of energy usage comes from distribution, because most of it is made overseas. Today, though there are silk factories in Europe and the Middle East, the leading silk industries are in China and India. Silk distribution to the US from China is around 5 billion kj.[15] This shows how the actual production of silk uses less energy than a synthetic material or cotton, but the majority of the energy used is due to distribution.
Production
Once the fabric is produced, it is distributed to various companies to be made into a variety of objects, like clothes, shoes, furniture, and more. On average, a textile mill including the textile machines as well as the electricity in the entire building is 2459 kW, or 2459 kJ/sec.[16]From there, consumers purchase the products that have taken so much energy to get to the final product. Velvet actually has a very long lifespan due to the way the fabric is looped and doesn’t take much energy to clean - only the occasional cycle in the washing machine for garments, which uses .5 kj/second. Not much energy goes into the use and reuse of velvet, ensuring a long life made to last.[17] When one is done with their velvet, it can be recycled and repurposed for a number of different things, which doesn’t require much energy at all because it’s repurposing the whole fabric as opposed to the specific elements of the item.
Conclusion
In conclusion, understanding of the energy and effort that goes into creating such a luxurious textile highlights the reasons why it is so highly regarded in the first place. It’s not just a fancy piece of fabric, it’s cotton that has been growing in the field and picked, or a silkworm across the world and 5 billion mj away, or petroleum that has been dug up from millions of years in the ground. So much energy is involved in the creation of this fabric that isn’t even thought about by the people it is being made for, all to sit aesthetically on a couch or as a fancy dress. However, though it does show how much effort goes into making such a material, the numbers attached to the embodied energy are scarily high. From just the amount of energy it takes to create the base fabric, ranging from 60 to nearly 300 mj, to then transport it, which, depending on the area of distribution can require up to 5 billion mj, to actually producing the velvet textile in factories, is it worth it? With the high numbers in energy comes a high number of byproducts, leaving a noticeable trail of toxins in the environment. However, just because the life cycle of velvet uses an excess of energy and releases waste into the environment, doesn’t mean that velvet should stop being in production. There are alternatives - for instance, cotton velvet takes a lot less energy in all aspects of the life cycle than the other materials. Being aware of the consequences and what it takes to create things shouldn’t be completely negative, instead it should spark the incentive to find cleaner, less energy intensive methods of making quality items.
Bibliography
“Camille.” Natural Clothing, 13 Dec. 2017, www.naturalclothing.com/what-is-polyamide-fabric-nylon-fabric/.
“From Cotton to Cloth.” National Museums Liverpool, www.liverpoolmuseums.org.uk/maritime/exhibitions/cotton/material/cloth.aspx.
“A Review of Energy Use and Energy Efficiency Technologies for the Textile Industry.” Renewable and Sustainable Energy Reviews, Pergamon, 27 Apr. 2012, www.sciencedirect.com/science/article/pii/S1364032112002122.
“Silk Making & Silk Production.” Silk Making; How to Make Silk; Silk Production Process; Silk Making Process; How Is Silk Made, texeresilk.com/article/silk_making_how_to_make_silk.
Silk-"The Queen of Fibers" - Watchtower ONLINE LIBRARY, wol.jw.org/en/wol/d/r1/lp-e/102006207.
“The Story of Cotton: How Cotton Is Grown, Processed, and Used.” Barnhardt Purified Cotton, 11 Jan. 2018, www.barnhardtcotton.net/blog/the-story-of-cotton-how-cotton-is-grown-processed-and-used/.
“A Technical Review of Emerging Technologies for Energy and Water Efficiency and Pollution Reduction in the Textile Industry.” Journal of Cleaner Production, Elsevier, 11 Mar. 2015, www.sciencedirect.com/science/article/pii/S095965261500205X.
“Velvet Material - 12 Different Types of This Beautiful Fabric.” Sew Guide, sewguide.com/velvet-material/.
Watt, Author: Melinda. “Renaissance Velvet Textiles | Essay | Heilbrunn Timeline of Art History | The Metropolitan Museum of Art.” The Met's Heilbrunn Timeline of Art History, www.metmuseum.org/toah/hd/velv/hd_velv.htm.
“What Is the Difference between Handmade Silk and Machine-Made Silk?” What Is the Difference between Handmade Silk and Machine-Made Silk? | Mizpah International Pty Ltd, www.mizpah.biz/what-difference-between-handmade-silk-and-machine-made-silk.
Boudreau, John. “Secrets of the Silk Worm: Inside a Factory in Cambodia.” Bloomberg.com, Bloomberg, 16 Nov. 2015, www.bloomberg.com/news/photo-essays/2015-11-16/secrets-of-the-silk-worm-inside-a-factory-in-cambodia.
“Cotton: from Field to Fabric.” Cotton: From Field to Fabric- Crop Production & Planting, www.cotton.org/pubs/cottoncounts/fieldtofabric/crops.cfm.
Dhayaneswaran, Y., and L. Ashokkumar. “A Study on Energy Conservation in Textile Industry.” Journal of The Institution of Engineers (India): Series B, vol. 94, no. 1, 2013, pp. 53–60., doi:10.1007/s40031-013-0040-5.
“Estimating the Carbon Footprint of a Fabric.” O ECOTEXTILES, 30 Jan. 2012, oecotextiles.wordpress.com/2011/01/19/estimating-the-carbon-footprint-of-a-fabric/.
“Fashion Archives: A Look at the History of Velvet.” StartUp FASHION, 4 Apr. 2016, startupfashion.com/fashion-archives-look-history-velvet/.
Khude, Prakash. “A Review on Energy Management in Textile Industry.” Innovative Energy & Research, vol. 06, no. 02, 2017, doi:10.4172/2576-1463.1000169.
“Life Cycle Analysis for Cotton and Polyester.” Sustainable Development, www.sustainability-ed.org.uk/pages/example4-3.htm.
“Story of Cotton.” Cotton's Journey, www.cottonsjourney.com/storyofcotton/page6.asp.
Cherrett, Nia, et al. “Ecological Footprint and Water Analysis of Cotton, Hemp, and Polyester.” Bioregional Development Group, 2005.
McIntyre, J. E. Synthetic Fibres: Nylon, Polyester, Acrylic, Polyolefin. Woodhead, 2005.
Contributors, HowStuffWorks.com. “How Much Fuel Does an International Plane Use for a Trip?” HowStuffWorks Science, HowStuffWorks, 28 Jan. 2015, science.howstuffworks.com/transport/flight/modern/question192.htm.
Wieck, Elizabeth. “The 7 Things You Need to Know About Velvet.” Materials Guide: 7 Things to Know About Velvet, 29 Sept. 2015, www.onekingslane.com/live-love-home/guide-to-velvet/.
[1] “Life Cycle Analysis for Cotton and Polyester.” Sustainable Development, www.sustainability-ed.org.uk/pages/example4-3.htm.
[2] “Cotton: from Field to Fabric.” Cotton: From Field to Fabric- Crop Production & Planting, www.cotton.org/pubs/cottoncounts/fieldtofabric/crops.cfm.
[3] “The Story of Cotton: How Cotton Is Grown, Processed, and Used.” Barnhardt Purified Cotton, 11 Jan. 2018, www.barnhardtcotton.net/blog/the-story-of-cotton-how-cotton-is-grown-processed-and-used/.
[4] “Estimating the Carbon Footprint of a Fabric.” O ECOTEXTILES, 30 Jan. 2012, oecotextiles.wordpress.com/2011/01/19/estimating-the-carbon-footprint-of-a-fabric/.
[5] “Story of Cotton.” Cotton's Journey, www.cottonsjourney.com/storyofcotton/page6.asp.
[6] “Estimating the Carbon Footprint of a Fabric.” O ECOTEXTILES, 30 Jan. 2012, oecotextiles.wordpress.com/2011/01/19/estimating-the-carbon-footprint-of-a-fabric/.
[7] “Camille.” Natural Clothing, 13 Dec. 2017, www.naturalclothing.com/what-is-polyamide-fabric-nylon-fabric/.
[8] “Estimating the Carbon Footprint of a Fabric.” O ECOTEXTILES, 30 Jan. 2012, oecotextiles.wordpress.com/2011/01/19/estimating-the-carbon-footprint-of-a-fabric/.
[9] Cherrett, Nia, et al. “Ecological Footprint and Water Analysis of Cotton, Hemp, and Polyester.” Bioregional Development Group, 2005.
[10] McIntyre, J. E. Synthetic Fibres: Nylon, Polyester, Acrylic, Polyolefin. Woodhead, 2005.
[11] Cherrett, Nia, et al. “Ecological Footprint and Water Analysis of Cotton, Hemp, and Polyester.” Bioregional Development Group, 2005.
[12] “Life Cycle Analysis for Cotton and Polyester.” Sustainable Development, www.sustainability-ed.org.uk/pages/example4-3.htm.
[13] “Silk Making & Silk Production.” Silk Making; How to Make Silk; Silk Production Process; Silk Making Process; How Is Silk Made, texeresilk.com/article/silk_making_how_to_make_silk.
[14] Boudreau, John. “Secrets of the Silk Worm: Inside a Factory in Cambodia.” Bloomberg.com, Bloomberg, 16 Nov. 2015, www.bloomberg.com/news/photo-essays/2015-11-16/secrets-of-the-silk-worm-inside-a-factory-in-cambodia.
[15] Contributors, HowStuffWorks.com. “How Much Fuel Does an International Plane Use for a Trip?” HowStuffWorks Science, HowStuffWorks, 28 Jan. 2015, science.howstuffworks.com/transport/flight/modern/question192.htm.
[16] Dhayaneswaran, Y., and L. Ashokkumar. “A Study on Energy Conservation in Textile Industry.” Journal of The Institution of Engineers (India): Series B, vol. 94, no. 1, 2013, pp. 53–60., doi:10.1007/s40031-013-0040-5.
[17] Wieck, Elizabeth. “The 7 Things You Need to Know About Velvet.” Materials Guide: 7 Things to Know About Velvet, 29 Sept. 2015, www.onekingslane.com/live-love-home/guide-to-velvet/.
Hanna Her
Dr. Christina Cogdell
DES040A
15 March 2018
Velvet: Wastes and Emissions
Known for its distinctive shine and texture--and historic symbolism for royalty and wealth—it’s no wonder why velvet is thought to be one of the most revered textiles to this day. However, we often forget--or are even unaware of--that producing this one-of-a-kind beauty is extremely costly: textile production is very energy-intensive and one of the greatest consumers of our finite raw materials, ultimately costing us our human, environmental, and economic health.
Because textiles are so highly integrated into our everyday products, it is unsurprising yet very concerning how underappreciated textile production is, especially since textile production accounts for 17-20% of all industrial wastewater[i] and 4% “of the secondary carbon footprint of an individual in the developed world” (Jain).
Throughout this report, I will explore the many by-products, wastes and emissions of velvet production. Velvet can be manufactured from many materials, yet for the sake of this report, I will narrow them down to the most common materials used: silk, cotton, synthetic fibers, and dyes. Because velvet is a secondary textile made from other textiles, it can be assumed that its waste and by-products are much more abundant than normal.
Therefore, through attempting to trace all of the waste generated in every step of the process down from the harvesting of raw materials to the reusable and non-reusable by-products, I hope to remove ignorance of the reality of velvet production and encourage consumers to be mindful of what they are purchasing.
Silk
Traditionally, velvet, according to URBANARA, is woven from silk[ii]. Silk is produced from the cocoons of the Bombyx Mori Moth larva, most commonly known as silkworms[iii]. Apart from the thousands of pupae boiled in the process of extracting the delicate fibers from the cocoons—in fact, it takes 2,000 to 3,000 cocoons to produce 1 pound of silk[iv]—25,425 kilograms of CO2 are released per tonne silk fiber[v]. What makes this statistic even more astonishing is the fact that velvet, as aforementioned, is manufactured from other textiles, suggesting that the scale of waste generated from production is much more concernedly high than if velvet was produced straight from the earth. Other organic waste include fibrous waste, which pollute waterways, and sericin gum, a by-product from the degumming process of the silk fibers[vi]. Sericin protein, fibroin, and microscopic silk fibers are also potential allergens to workers[vii].
Not all hope is lost, fortunately, as the dead pupae are used as viable sources of food, sericin gum for cosmetics and pharmaceutical use[vi] and pupae oil for “paints, varnishes, pharmaceuticals, soaps, candles, plastic, and biofuels” (qtd. in Javali et al.). The silk fibrous waste is used to extract silk fibroin, a protein that is useful for “films, porous scaffolds and nonwovens for various biomedical applications” (Gaviria et al.).
However, extracting the silk fibroin is also energy-intensive as the whole process includes more degumming, production of wastewater, dissolving the fibroin in formic acid, and actually extracting the silk fibroin from the formic acid solution via electrospinning[viii].
I could not find information on the very unusable by-products of silk but can infer that not all waste is re-circulated back into industry due to the amount of energy to recycle material and the waste quality needed in order to be recycled. I also couldn’t find information on the amount of silk that ends up in landfills and incinerators. General statistics on textile waste, however, suggests that silk and silk velvet waste makes up a significant percentage of the millions of tons of textiles just simply thrown away and left to rot.
Therefore, we can assume that waste from silk production will never be completely eradicated; however, each effort to minimize waste can definitely help make the production process more environmentally-friendly for silk and for velvet.
Cotton
Today, velvet is also produced from cotton. Cotton production is responsible for 0.3% to 1% of the total global greenhouse gas emissions[ix] and is one of the world’s greatest culprits of generating wastewater. According to The Guardian, “the global average water footprint for 1kg of cotton is 10,000 litres” (Leahy).
At the plantation stage, the cotton plant already “consumes 10% of all agricultural chemicals and 25% of insecticides,” much of which gets into nearby waterways and harms ecosystems that depend on that water or on nearby land (Sweeny). In the U.S., research done in the 1990’s reported that pesticides were present in all samples from major rivers and that about 90% of water and fish samples from every tested water sample contained pesticides[x]. Pesticide poisoning also affects about a million people per year through death or illness and disease[x]; health problems include “vomiting, fatigue …psychological, neurological, cardiorespiratory and gastrointestinal symptoms coupled with low plasma ChE activity” (Aktar et al.). Other substances released from cotton production include cotton dust, endotoxin, bacteria and silica dust, all which cause many respiratory problems if inhaled[xi].
Besides all of these health-related issues, there is fortunately a light at the end of this road. Most of the by-products from the cotton production process are recycled. The most common cotton by-products are seeds, oil, and hulls, which are reused for paper, feed, cooking, and cosmetics; after a process known as “ginning,” the fuzz—known as linter—is employed for medical purposes and gunpowder[xii].
I could not find much about the use of cotton to produce velvet fabric, but I assume the velvet production process stays the same no matter what raw material is used with only some slight differences.
Synthetics
Synthetic textiles are notorious for their inability to biodegrade and their increasing popularity in today’s products. For the manufacturing of velvet, the most common synthetic textiles used include nylon, acetate, polyester, and rayon.
Despite the heavy variety of synthetic textiles, all (except for rayon, which comes from cellulose[xiii]) stem from one source: crude oil.
It is already common knowledge that plastics—synthetic fabrics are plastic fabric—do not biodegrade and will take millions of years to do so. During production, plastic fibers are shed and make their way into our waters, our lungs, and into the rest of our environment[xi]. But let’s take a step back and examine the wastes and by-products released at the start of synthetic textile production: harvesting the oil. Crude oil is harvested by drilling the earth to extract the oil. Waste released includes drilling fluids and drilling muds, cuttings, toxic gas, and even radioactive waste such as uranium, thorium, and radium[xiv]. One of the most prominent drilling fluid wastes, called “produced water,” is according to the EPA “water pumped from wells and separated from the oil and gas produced [where] radioactivity levels…from unconventional drilling can be significant” (“TENORM”). Produced water contains many radioactive wastes, particularly radium, and is either buried deep under the ground or treated into something reusable[xiv]. One very saddening statistic from the American Petroleum Institute states that “more than 18 billion barrels of waste fluids from oil and gas production are generated annually in the United States” (qtd. in “TENORM”). Other statistics from EPA’s article include that about 100 tons of scale per oil well and 5 million cubic feet of sludge are produced annually from oil drilling[xiv]. Although much of the by-products and waste that come from oil drilling cannot be reused, sulfur is. Sulfur is utilized for fertilizers, cellophane, viscose, and most relevantly the production of rayon[xv].
During the actual textile production process, the polymers that come from the crude oil are spun into fibers via wet spinning, dry spinning, and melt spinning; each process is a different variation of forcing the polymer fluid through spinnerettes to form the fibers. According to the EPA, each spin process releases similar wastes such as solvent and organic waste; dry spinning is particularly known for releasing large amounts of VOCs (volatile organic compounds), wet spinning for solvent waste and emissions and melt spinning for aerosol emissions[xvi]. The solvent used in the fiber production process are recovered at 94-98% while VOCs are not usually recovered[xvi]. At the end of synthetic fabric’s life cycle, the textile is either thrown into landfills and incinerators or recycled by being shredded, turned into chips, melted, and re-spun into new fibers[xvii].
Dyes
Velvet comes in many colors ranging from a rich black to the lightest pink. To produce colorful velvet, or colorful textiles in general, industry uses dyes which can be either natural or synthetic. I could find no statistics on whether synthetic or natural dyes are used more often but due to synthetic dyeing’s overwhelming popularity in industry today—since it is inexpensive, long-lasting, and more efficient than natural dyes—it can be assumed that most of the dye pollution stems from synthetic dyes.
To produce plant dyes, large amounts of land are needed. For example, “13 acres of land is needed to grow enough dye for one acre of cotton” including all of the fertilizers and pesticides used as well (Chhabra). Furthermore, mordants must often be used to bind natural dye to the fabric which results in heavy metal waste and acids[xviii]. To extract colors from natural sources, plant, animal, and earthen material undergo an energy-intensive process involving drying and crushing the material, using solvents to extract compounds, and further separating these compounds to get the desired dyes[xix]; this process involves large amounts of wastewater, toxic solvents, and release of greenhouse gas emissions[xx]. Thus, though natural dyes are usually seen as more sustainable and environmentally-friendly than synthetic dyes, the processes to extract them still release a significant amount of waste.
And if natural dyes can cause some damage, synthetic dyes do even more. In industry 10,000 and counting dyes and pigments are produced annually and around 200,000 tons of dyes become discharge that then infiltrates our environment[xxi]. Waterborne wastes include chlorine, sulfides, metals, acids, salts, and pigments. More chemicals released include acetic acid, ammonium sulphate, caustic soda and others[xxii]. Some are so carcinogenic and hazardous that they are heavily regulated, such as “tributyltin (TBT), pentabromodiphenyl ether (PBDE), phthalates, perfluorooctane sulphonate (PFOS), and aniline” (Heida). In addition, the pigments are also made to be permanent and thus do not biodegrade easily[xvii].
Because of the large amounts of wastewater, there are many attempts to treat it such as through incineration and with bacteria, although many of the methods prove to be ineffective due to the complexity of the effluents, incomplete combustion, generation of sludge, and long biological treatment time[xxi].
Transportation and Other Wastes
To obtain all of the raw materials needed to produce velvet, which are grown all over the world, we must transport them to textile mills via truck, ship, or plane.
In regards to cotton transportation specifically, trucks emit 1.15 pounds of carbon dioxide per mile[xxiii]. I couldn’t find data on the amount of waste released transporting the other raw materials but I assume that their carbon footprint would be about the same due to how vital they are to velvet production. In general, for the U.S., transportation—all kinds of transportation, not just for textile/raw material transportation—accounts for about 30% of all U.S. greenhouse gas emissions; because of the scale of the textile industry, transporting textiles/raw materials alone makes up a significant part of that 30%[xxiv]. Other emissions and by-products include carbon monoxide, nitrogen oxides, hydrocarbons, and ozone[xxv]. According to European-based Transport and Environment, ships emit 1.7 million tons of sulfur dioxide, 2.8 million tons nitrogen dioxide, and 195,000 tons of particulate matter annually, again suggesting the giant role the textile industry plays in those statistics[xxvi]. This is the price that the textile industry—and even we—must pay in order to continue consuming as much as we do.
Conclusion
It is a very imperative matter that we consumers become educated about the colossal amount of wastes and by-products that come out of not just velvet production but all textile production. If we continue to remain ignorant, intentionally or not, we will surely consume every last bit of finite raw material that comes from this earth. As consumers, we must change our spending habits and learn either to cut back on consumption or find more sustainable alternatives that will both save our planet and be economically feasible for our society.
[i] Kant, R. (2012) Textile dyeing industry an environmental hazard. Natural Science, 4, 22-26. doi: 10.4236/ns.2012.41004.
[ii] “Velvet.” URBANARA Journal, URBANARA, 11 Nov. 2015, www.urbanara.co.uk/journal/buying-guide/velvet/.
[iii] Lin, Doris. “Why Vegans Don't Wear Silk.” ThoughtCo, ThoughtCo, 16 July 2017, www.thoughtco.com/why-vegans-dont-wear-silk-127729.
[iv] Rowland, Teisha. “Turning Leaves Into Silk.” Turning Leaves Into Silk, Santa Barbara Independent, Inc., 12 Mar. 2010, www.independent.com/news/2010/mar/12/turning- leaves-silk/.
[v] A M Giacomin et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 254 192008
[vi] Javali, U.C., et al. “Developments in the Use of Silk by-Products and Silk Waste.” Advances in Silk Science and Technology, ser. 163, 8 May 2015, pp. 261–270. ScienceDirect, doi:10.1016/b978-1-78242-311-9.00013-6.
[vii] Gowda G, Shivalingaiah AH, Vijayeendra AM, Sarkar N, Nagaraj C, Masthi NR. Sensitization to silk allergen among workers of silk filatures in India: a comparative study. Asia Pac Allergy. 2016 Apr;6(2):90- 93.https://doi.org/10.5415/apallergy.2016.6.2.90
[viii] A Gaviria et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 254 102005
[ix] International Trade Centre (ITC). “Cotton and Climate Change: Impacts and Options to Mitigate and Adapt.” International Trade Centre, 11 Mar. 2011.
[x] Aktar, Wasim, Dwaipayan Sengupta and Ashim Chowdhury. "Impact of pesticides use in agriculture: their benefits and hazards" Interdisciplinary Toxicology, 2.1 (2009): 1-12. Retrieved 6 Mar. 2018, from doi:10.2478/v10102-009-0001-7
[xi] Dutkiewicz J, Mackiewicz B, Lemieszek MK, Golec M, Milanowski J. “Pantoea agglomerans: a marvelous bacterium of evil and good. Part I. Deleterious effects: Dust-borne endotoxins and allergens—focus on cotton dust.” Annals of agricultural and environmental medicine: AAEM. 2015;22(4):576–88. Epub 2015/12/29. doi: 10.5604/12321966.1185757 . [PubMed]
[xii] “Cotton Byproducts.” Cotton Today, COTTON INCORPORATED, cottontoday.cottoninc.com/cotton-byproducts/.
[xiii] Mass, Ed. “Eco-Fiber or Fraud? Are Rayon, Modal, and Tencel Environmental Friends or Foes?.” Natural Life Magazine, Life Media, 2009, www.life.ca/naturallife/0908/ecofiber_or_fraud.htm.
[xiv] “TENORM: Oil and Gas Production Wastes.” EPA, Environmental Protection Agency, 31 Oct. 2017, www.epa.gov/radiation/tenorm-oil-and-gas-production-wastes.
[xv] Sosnowska, Monika. “Crude Oil and Its Derivatives.” MINTEC, Mintec Limited, 30 May 2012, www.mintecglobal.com/newsletter/crude-oil-and-its-derivatives.
[xvi] United States Environmental Protection Agency. “6.9 Synthetic Fibers.” AP-42: Compilation of Air Emission Factors, 5th ed., vol. 1, pp. 1–90.
[xvii] LeBlanc, Rick. “The Basics of Clothing and Textile Recycling.” The Balance, The Balance, 1 Mar. 2017, www.thebalance.com/the-basics-of-recycling-clothing-and-other-textiles-2877780.
[xviii] Chhabra, Esha. “Natural Dyes v Synthetic: Which Is More Sustainable?” The Guardian, Guardian News and Media, 31 Mar. 2015, www.theguardian.com/sustainable-business/sustainable-fashion-blog/2015/mar/31/natural-dyes-v-synthetic-which-is-more-sustainable.
[xix] Yusuf, Mohd, Mohd Shabbir, and Faqeer Mohammad. “Natural Colorants: Historical, Processing and Sustainable Prospects.” Natural Products and Bioprospecting 7.1 (2017): 123–145. PMC. Web. 11 Mar. 2018.
[xx] Saxena, Sujata, and A.S.M. Raja. “Natural Dyes: Sources, Chemistry, Application and Sustainability Issues.” Textile Science and Clothing Technology, Springer Singapore, 2014, 37-80. Crossref, doi:10.1007/978-981-287-065-0_2.
[xxi] Farah Maria Drumond Chequer, Gisele Augusto Rodrigues de Oliveira, Elisa Raquel Anastácio Ferraz, Juliano Carvalho Cardoso, Maria Valnice Boldrin Zanoni and Danielle Palma de Oliveira (2013). Textile Dyes: Dyeing Process and Environmental Impact, Eco-Friendly Textile Dyeing and Finishing, Dr. Melih Gunay (Ed.), InTech, DOI:10.5772/53659.
[xxii] Kant, R. (2012) Textile dyeing industry an environmental hazard. Natural Science, 4, 22-26. doi: 10.4236/ns.2012.41004.
[xxiii] Wallander, Mattias. “The Lifecycle of a T-Shirt.” The Huffington Post, TheHuffingtonPost.com, 30 June 2011, www.huffingtonpost.com/mattias-wallander/the-lifecycle-of-a-tshirt_b_887133.html.
[xxiv] “Car Emissions and Global Warming.” Union of Concerned Scientists, Union of Concerned Scientists , www.ucsusa.org/clean-vehicles/car-emissions-and-global- warming#.WqX5rOjwbDd.
[xxv] “Vehicle Emissions Testing.” Illinois Environmental Protection Agency, Illinois EPA, www.epa.illinois.gov/topics/air-quality/mobile-sources/vehicle-emissions-testing/index.
[xxvi] “Air Pollution from Ships.” Transport and Environment , European Federation for Transport and Environment AISBL, www.transportenvironment.org/what-we-do/shipping/air-pollution-ships.
Bibliography
A Gaviria et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 254 102005
Aktar, Wasim, Dwaipayan Sengupta and Ashim Chowdhury. "Impact of pesticides use in agriculture: their benefits and hazards" Interdisciplinary Toxicology, 2.1 (2009): 1-12. Retrieved 6 Mar. 2018, from doi:10.2478/v10102-009-0001-7
A M Giacomin et al 2017 IOP Conf. Ser.: Mater. Sci. Eng. 254 192008
“About Velvet.” Thevelvetlab, Thevelvetlab, www.thevelvetlab.com/topic/26-about-velvet.aspx.
“Air Pollution from Ships.” Transport and Environment , European Federation for Transport and Environment AISBL, www.transportenvironment.org/what-we-do/shipping/air-pollution-ships.
Aragonés-Beltrán, P., et al. "Application of Multicriteria Decision Analysis to Jar-Test Results for Chemicals Selection in the Physical-Chemical Treatment of Textile Wastewater." Journal of Hazardous Materials, vol. 164, no. 1, 2009, pp. 288-295, Toxline, https://search.proquest.com/docview/67006216?accountid=14505, doi:http://dx.doi.org/10.1016/j.jhazmat.2008.08.046.
“Car Emissions and Global Warming.” Union of Concerned Scientists, Union of Concerned Scientists , www.ucsusa.org/clean-vehicles/car-emissions-and-global- warming#.WqX5rOjwbDd.
Chhabra, Esha. “Natural Dyes v Synthetic: Which Is More Sustainable?” The Guardian, Guardian News and Media, 31 Mar. 2015, www.theguardian.com/sustainable- business/sustainable-fashion-blog/2015/mar/31/natural-dyes-v-synthetic-which-is-more-sustainable.
Claudio, Luz. “Waste Couture: Environmental Impact of the Clothing Industry.” Environmental Health Perspectives 115.9 (2007): A449–A454. Print.
“Cotton Byproducts.” Cotton Today, COTTON INCORPORATED, cottontoday.cottoninc.com/cotton-byproducts/.
Diguer, Michel, et al. Velvet. Patente Et Bidule, Making Stuff Productions, 2010, www.tfo.org/en/universe/patente-and-bidule/100261126/velvet.
Dutkiewicz J, Mackiewicz B, Lemieszek MK, Golec M, Milanowski J. “Pantoea agglomerans: a marvelous bacterium of evil and good. Part I. Deleterious effects: Dust- borne endotoxins and allergens—focus on cotton dust.” Annals of agricultural and environmental medicine: AAEM. 2015;22(4):576–88. Epub 2015/12/29. doi: 10.5604/12321966.1185757 . [PubMed]
Farah Maria Drumond Chequer, Gisele Augusto Rodrigues de Oliveira, Elisa Raquel Anastácio Ferraz, Juliano Carvalho Cardoso, Maria Valnice Boldrin Zanoni and Danielle Palma de Oliveira (2013). Textile Dyes: Dyeing Process and Environmental Impact, Eco-Friendly Textile Dyeing and Finishing, Dr. Melih Gunay (Ed.), InTech, DOI: 10.5772/53659.
“FROM FLAX TO LINEN.” Libeco, Masters of Linen, www.libeco.com/en/about-linen/from- flax-to-linen.aspx.
Gowda G, Shivalingaiah AH, Vijayeendra AM, Sarkar N, Nagaraj C, Masthi NR. Sensitization to silk allergen among workers of silk filatures in India: a comparative study. Asia Pac Allergy. 2016 Apr;6(2):90- 93.https://doi.org/10.5415/apallergy.2016.6.2.90
Heida, Lydia. “Can Waterless Dyeing Processes Clean Up the Clothing Industry?” Yale E360, Yale School of Forestry and Environmental Studies, 12 June 2014, e360.yale.edu/features/can_waterless_dyeing_processes_clean_up_clothing_industry_pol lution.
International Trade Centre (ITC). “Cotton and Climate Change: Impacts and Options to Mitigate and Adapt.” International Trade Centre, 11 Mar. 2011.
Jain, Minakshi (2017). Ecological approach to reduce carbon footprint of textile industry. Internat. J. Appl. Home Sci., 4 (7 & 8) : 623-633.
Javali, U.C., et al. “Developments in the Use of Silk by-Products and Silk Waste.” Advances in Silk Science and Technology, ser. 163, 8 May 2015, pp. 261–270. ScienceDirect, doi:10.1016/b978-1-78242-311-9.00013-6.
Kant, R. (2012) Textile dyeing industry an environmental hazard. Natural Science, 4, 22-26. doi: 10.4236/ns.2012.41004.
Kumar, S. S., and K. Akil. "A Novel Eco-Friendly Approach for Textile Sludge."Journal of Environmental Science & Engineering, vol. 48, no. 1, 2006, pp. 9-14, Toxline, https://search.proquest.com/docview/68355862?accountid=14505.
LeBlanc, Rick. “The Basics of Clothing and Textile Recycling.” The Balance, The Balance, 1 Mar. 2017, www.thebalance.com/the-basics-of-recycling-clothing-and-other-textiles- 2877780.
Lin, Doris. “Why Vegans Don't Wear Silk.” ThoughtCo, ThoughtCo, 16 July 2017, www.thoughtco.com/why-vegans-dont-wear-silk-127729.
Mass, Ed. “Eco-Fiber or Fraud? Are Rayon, Modal, and Tencel Environmental Friends or Foes?.” Natural Life Magazine, Life Media, 2009, www.life.ca/naturallife/0908/ecofiber_or_fraud.htm.Nasrullah, Muhammad, et al. "Elemental Balance of SRF Production Process: Solid Recovered Fuel Produced from Municipal Solid Waste." Waste Management & Research: The Journal of the International Solid Wastes and Public Cleansing Association, ISWA, vol. 34, no. 1, 2016, pp. 38-46, Toxline, https://search.proquest.com/docview/1749983407?accountid=14505, doi:http://dx.doi.org/10.1177/0734242X15615697.
Pala, A. "Chemical Treatment of Textile Wastewaters: Statistical Characterization, Colour and Sulfide Removal." Indian Journal of Environmental Health, vol. 43, no. 3, 2001, pp.128-134, Toxline, https://search.proquest.com/docview/71334465?accountid=14505.
Ross, Charlie. “How Silk Fabric Comes To Life: Meeting Our Silk Moths.” The Swatch Book, Offset Warehouse, 19 Aug. 2015, theswatchbook.offsetwarehouse.com/2015/08/18/how-is-silk-fabric-made/.
Ross, Charlie. “What Synthetic Materials Are Doing To Our Environment.” The Swatch Book, Offset Warehouse, 10 Apr. 2017, theswatchbook.offsetwarehouse.com/2017/04/11/synthetic-materials-environment/.
Rowland, Teisha. “Turning Leaves Into Silk.” Turning Leaves Into Silk, Santa Barbara Independent, Inc., 12 Mar. 2010, www.independent.com/news/2010/mar/12/turning-leaves-silk/.
Saxena, Sujata, and A. S. M. Raja. “Natural Dyes: Sources, Chemistry, Application and Sustainability Issues.” Textile Science and Clothing Technology, Springer Singapore, 2014, 37–80. Crossref, doi:10.1007/978-981-287-065-0_2.
Sharma, K. P., et al. "A Comparative Study on Characterization of Textile Wastewaters (Untreated and Treated) Toxicity by Chemical and Biological Tests." Chemosphere, vol. 69, no. 1, 2007, pp. 48-54, Toxline, https://search.proquest.com/docview/68136578?accountid=14505.
Sosnowska, Monika. “Crude Oil and Its Derivatives.” MINTEC, Mintec Limited, 30 May 2012, www.mintecglobal.com/newsletter/crude-oil-and-its-derivatives.
Sweeny, Glynis. “It's the Second Dirtiest Thing in the World-And You're Wearing It.” Alternet, Alternet, 13 Aug. 2015, www.alternet.org/environment/its-second-dirtiest-thing-world-and-youre-wearing-it.
“TENORM: Oil and Gas Production Wastes.” EPA, Environmental Protection Agency, 31 Oct.2017, www.epa.gov/radiation/tenorm-oil-and-gas-production-wastes.
Tiwari, Meenaxi and Babel, Sudha (2013). Air Pollution in Textile Industry. Asian J. Environ. Sci., 8 (1): 64-66.
United States Environmental Protection Agency. “6.9 Synthetic Fibers.” AP-42: Compilation of Air Emission Factors, 5th ed., vol. 1, pp. 1–90.
“US Dollars Spent on Cotton Pesticides.” The World Counts, The World Counts, www.theworldcounts.com/counters/cotton_environmental_impacts/cotton_pesticides_statistics.
“Vehicle Emissions Testing.” Illinois Environmental Protection Agency, Illinois EPA, www.epa.illinois.gov/topics/air-quality/mobile-sources/vehicle-emissions-testing/index.
“Velvet.” URBANARA Journal, URBANARA, 11 Nov. 2015, www.urbanara.co.uk/journal/buying-guide/velvet/.
Wallander, Mattias. “The Lifecycle of a T-Shirt.” The Huffington Post, TheHuffingtonPost.com, 30 June 2011, www.huffingtonpost.com/mattias-wallander/the-lifecycle-of-a-tshirt_b_887133.html.
Watt, Melinda. “Renaissance Velvet Textiles | Essay | Heilbrunn Timeline of Art History | The Metropolitan Museum of Art.” The Met's Heilbrunn Timeline of Art History, The Metropolitan Museum of Art, Aug. 2011, www.metmuseum.org/toah/hd/velv/hd_velv.htm.
Wernli, Karen J., et al. "Occupational Exposures and Ovarian Cancer in Textile Workers." Epidemiology (Cambridge, Mass.), vol. 19, no. 2, 2008, pp. 244-250, Toxline, https://search.proquest.com/docview/68566225?accountid=14505, doi:http://dx.doi.org/10.1097/EDE.0b013e31816339f9.
“Where Is It Grown?” Cotton Australia, T-Bone, cottonaustralia.com.au/australian-cotton/basics/where-is-it-grown.
World Bank. (2010). Introduction to Greenhouse Gas Emissions in Road Construction and Rehabilitation [Executive Summary]. Greenhouse Gas Emissions Mitigation in Road Construction and Rehabilitation.
Yusuf, Mohd, Mohd Shabbir, and Faqeer Mohammad. “Natural Colorants: Historical, Processing and Sustainable Prospects.” Natural Products and Bioprospecting 7.1 (2017): 123–145. PMC. Web. 11 Mar. 2018.