Design Life-Cycle
assess.design.(don't)consume
Aidan Malmberg
Cogdell
DES040A 001
25 February 2018
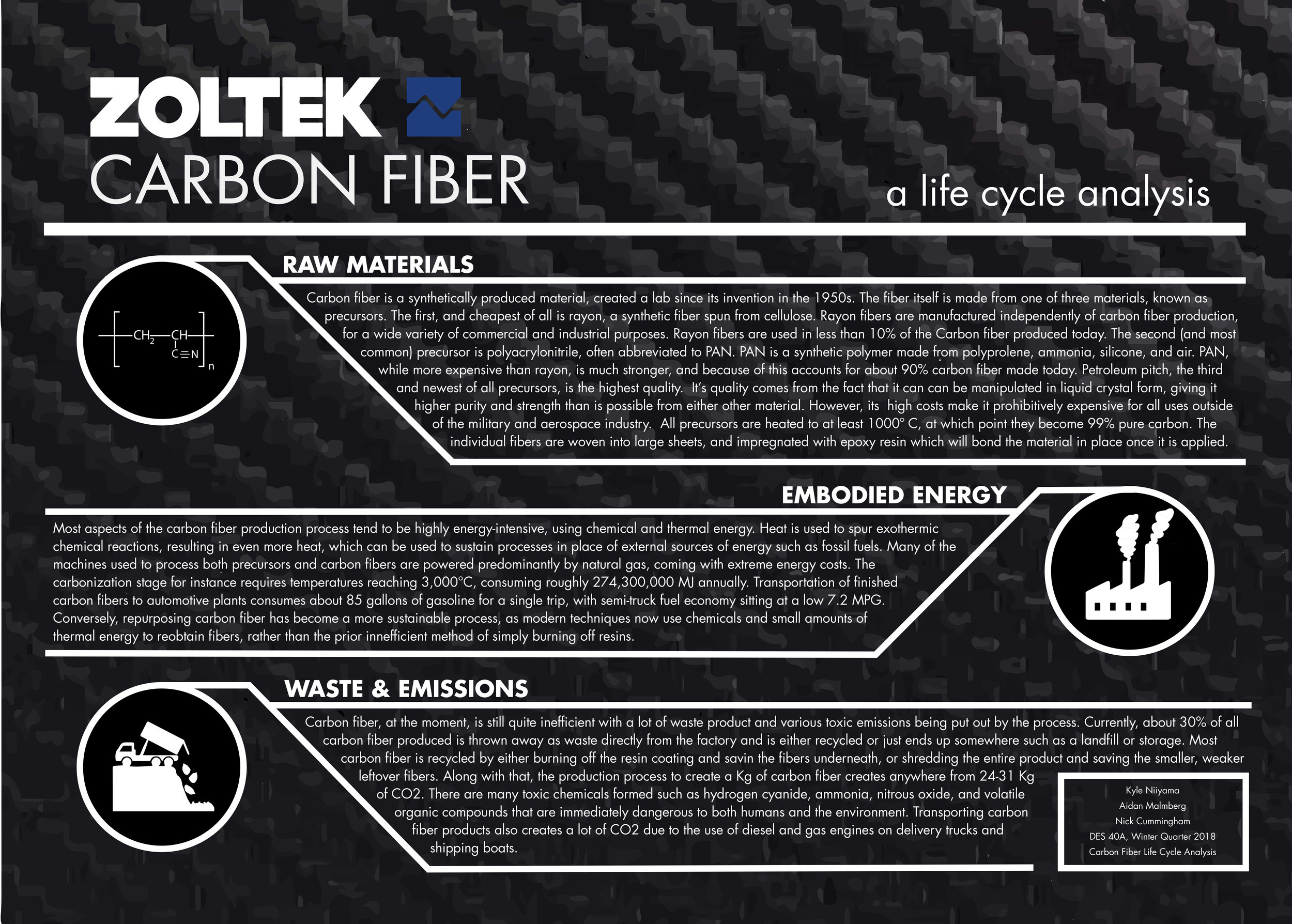
Raw Materials in Carbon Fiber
Carbon fiber is a relatively new material in the world of design and engineering, but it has seemingly revolutionized the market. Because of its visible highly visible use within the aerospace industry in spacecraft and high-performance aircraft, the material has developed a certain prestige which has trickled down to much more everyday uses. The true benefits of carbon fiber lie not in the prestige of it’s applications, but it’s very unique set of properties. Carbon fiber is extremely lightweight, and be made to be as stiff or flexible as desired, can be both easily shaped like cloth and molded like plastic, and is highly temperature resilient. It’s unique and versatile properties have created a much higher demand for the material than there is a supply of. Although carbon fiber has historically been inefficient to source materials for, recent developments in the refinement and reclamation process have allowed carbon fiber to become more and more efficient and environmentally friendly to produce, and although the initial material sourcing is resource-intensive, current trends in material reclamation have slowed that course.
How has the one of the most common elements come to be the foundation for one of the most valued and high-performance materials available to designers today? Although material itself is still young, the technology and concept came from two much older industries- textiles and energy. The first individual carbon fibers were created as a byproduct of the lighting industry. Small strips of carbon were used in carbon arc lamps in the late 1800s, although creation of the first “carbon fiber” is credited to Thomas Edison ("High Performance Carbon Fibers”). This event occurred as Edison experimented with filaments for incandescent bulbs, when he accidentally carbonized a cotton fiber and discovered it had much more durable qualities that the original. However, It was not until 80 years later in the technological boom of postwar America that carbon fiber developed much beyond. Dr. Roger Bacon, a physicist at Union Carbide, discovered that graphite, a highly refined form of carbon, forms in perfectly hexagonal sheets, which can be rolled into individual strands no more than the width of a molecule across, less than a tenth the width of human hair. These microtubules had a tensile strength of 20 GigaPascals (GPa), and a Young’s modulus of 700 GPa. These values were ten and three times the strength of steel at the time, respectively. Unfortunately, costs for the new wonder material ran as high as $10 million per pound, making it prohibitively expensive for any practical use in the 1950s. Much of the research since then has been aimed at reducing that cost.
As is a lab-created, manufactured material, each brand has their own formula and technique for carbon manufacturing, meaning each individual brand has its own individual characteristics which are favored by different consumers. Unfortunately the many brands keep their specific formulas closely guarded, as they are considered to be a trade secret(“How Is Carbon Fiber Made?”). Still, among every brand, the principles remain roughly the same. The single largest cost in carbon manufacturing is the initial expense of the facility. A world class carbon fiber manufacturing plant can cost upwards of $25 million dollars (McConnell). Much of the costs of the carbon itself are involved in offsetting the high costs of establishing and operating a lab.
The most obvious component in rough carbon fiber sheets is the one from which it takes its name, the actual carbon fiber, known as the precursor. The precursor is generally made from one of three materials: rayon, pitch, or polyacrylonitrile (usually abbreviated to PAN). Rayon, a cellulose-based artificial fiber, was what was originally used by Bacon in his experiments at United Carbide, and still accounts for about 10% of all carbon fiber production(McConnell). Rayon fibers are manufactured independently of carbon fiber production, for a wide variety of commercial and industrial purposes (Rayon). It is important to be aware of rayon production sources, however, as cellulose and rayon filaments production plants can be significant sources of pollution and are often located in developing nations. Rayon production relies heavily on the use of industrial sulfuric acid, which can be detrimental to the employees and environments of production plants.
Although carbon fiber had first been developed in the United States using rayon fibers, Japan and England soon began utilizing polyacrylonitrile for their carbon projects. United Carbide initially attempted to use PAN for their carbonization processes, but was unsuccessful due to the unavailability of high quality polyacrylonitrile In the U.S. ("High Performance Carbon Fibers”). Overseas, however, the British and Japanese had unlimited access to the material. PAN is a polymer of acrylonitrile, a monomer, a chemical produced with, propylene, ammonia, and air as an oxidizing agent. Although it was formerly sourced from elsewhere in the past, today is generally synthesized on-site specifically for the purpose of carbon production. This is because it the main use of PAN is carbon production, and it is more environmentally and logistically efficient to produce it on site, cutting transportation costs. Silicone is added to the material during the polymerization process, seeing as a non-binding agent to keep the fibers from adhering to one-another (“Polyacrylonitrile (PAN): How is it Made?”). The fibers are then wounds, stretched, and spun to a diameter of 10 to 20µm to complete the precursor manufacturing process. While PAN fibers are much more useful than rayon fibers, being both stronger and lighter, is is much more expensive to use as a precursor, costing about $15 per pound. Additionally, the environmental costs of procuring the pure materials required to synthesize PAN are quite high, as producers are reliant on mining and chemical refineries to supply the monomer to them.
The final type of precursor, pitch, is the newest, highest quality, and least used. Pitch is a distilled organic compound, created from highly refined plant matter, oil, or coal, though oil is the most common (“How Is Carbon Fiber Made?”, Matsumoto). While pitch is naturally multidirectional in structure, research by Australian scientists found a means of refining it slightly further and making it layered and unidirectional in form. The reason pitch is such high quality is the ability for scientists to manipulate it in mesophase, or a liquid crystal state. As a result of this, however, it is extremely expensive, and is currently only practical for use in aerospace and military capacities(Das).
Regardless of which precursor is used, all fibers still need to be carbonized before they can be woven into carbon sheets. Precursors must be heated to over 1000º celsius to achieve a usable 99% purity, and can reach 100% purity after being heated to over 3000º C (“How Is Carbon Fiber Made?”). Out of PAN, rayon, and pitch, only some pitch can be graphitized. This is because only come pitch starts with with a pure enough structure to become graphite.
After carbonization, the carbon fibers are woven into sheets, and impregnated with resin which can be used to solidify the material once the the sheet is applied. The resins used are most commonly are epoxies, which are set chemically with a curing agent or thermally in an oven(Sloan). Epoxies are a petroleum-derived industrial adhesive widely used across many industries, and sets up like a plastic. When the resin is set, the sheet is locked in place, and the stiffness of the resin is reinforced by the strength of the carbo fibers. These complementary traits are what makes carbon fiber such a versatile material.
While carbon is very resource-intensive to initially manufacture, work is being done to increase the rate of carbon-fiber recycling. Due to the complexity of the material, recycling carbon has historically been a challenge, but companies in Europe and the United States have recently developed a handful of new methods. An especially promising development has been the process of incinerating carbon sheeting in an oxygen-poor environment, removing the resin and yielding the carbon fibers exactly as they were(Rush). Other companies are working to develop resins that can be chemically or chemically remolded, allowing for the repurposing of fibers in a select set of conditions (Harris). A third method involves shredding old carbon sheeting and re-weaving it, though this can only be done for lower-grade carbon, as the fibers do not retain much strength through the shredding process. The ability to improve and expand carbon recycling in the future is important, and could lead to lower costs in the future. Improved recycling processes will also help limit reduce the high environmental costs of carbon manufacturing, from eliminating the steps of materials acquisition and processing form the manufacturing process.
While carbon fiber is a very versatile and high-performance material with applications from skyscrapers to hockey sticks, it has an enormous cost in materials, energy, and waste. The materials required to make carbon are highly highly refined, and the process involves many harmful chemicals and emissions. Thankfully, carbon lasts a very long time, and the process is always developing, and will continue to get more and more efficient as time goes on. This material is still comparatively very young, and it will be interesting to see how it develops in the coming years.
Works Cited
Das, et al. “Global Carbon Fiber Composites Supply Chain Competitiveness Analysis.” Department of Energy, energy.gov/.
Harris, Mark. “Carbon Fiber: the Wonder Material with a Dirty Secret.” The Guardian, Guardian News and Media, 22 Mar. 2017, www.theguardian.com/.
"High Performance Carbon Fibers."American Chemical Society National Historic Chemical Landmarks, 12 March 2018. http://www.acs.org/.
“How Is Carbon Fiber Made?” ZOLTEK, Toray Corporation, 23 Oct. 2017, zoltek.com/.
Matsumoto, T. "Mesophase pitch and its carbon fibers" (PDF). Pure and Applied Chemistry.1995.
McConnell, Vicki. “The Making of Carbon Fiber.” CompositesWorld, CompositesWorld, 18 Dec. 2008, www.compositesworld.com/.
“Polyacrylonitrile (PAN): How Is It Made? - Toray CFE, Fibres De Carbone Et Composites.” Toray-CFE, Toray Carbon Fibers, Europe, 13 Mar. 2018, www.toray-cfe.com/.
“Rayon.” American Fiber Manufacturers Association, American Fiber Manufacturers Association, 2017, www.fibersource.com/.
Rush, Susan. “Carbon Fiber: Life Beyond the Landfill.” CompositesWorld,
Sloan, Jeff. “Composites 101: Fibers and Resins.” CompositesWorld, CompositesWorld, 14 Mar. 2016, www.compositesworld.com/.
"What Is Carbon Fiber?” Innovative Composite Engineering, 20 Jan. 2015, www.innovativecomposite.com/.
Kyle Niiyama
Cogdell
DES 40A
4 March 2018
The Embodied Energy of Carbon Fiber
Carbon fiber is the building material of the future. Over the last thirty years, the fibers have become increasingly prominent in modern consumerism, seen in a variety of products ranging from aerospace components to automobiles to bike frames, and now even sports equipment. But what makes carbon fiber so special? The answer lies in the fibers’ extremely high strength to weight ratio. For instance, carbon fiber may be up to ten times as strong and five times as light as steel and eight times stronger and almost two times lighter than aluminum, which are already considered to be strong, durable building materials [1]. However, such a seemingly perfect product is not without its drawbacks. While ZOLTEK, one of the world’s leading manufacturers of carbon fiber, has made impressive strides in innovation and attempted to improve efficiency by using more advanced production equipment, carbon fiber’s energy cost still remains significantly high.
Carbon fiber itself consists of a few simple raw materials, known as precursors. The most common carbon fibers are constructed using polyacrylonitrile, or PAN, a chemical mixture containing ammonia, propylene, and oxygen [2]. I was unable to find the exact PAN manufacturing techniques used by ZOLTEK, so instead I researched standard methods used in the industry. Most industrial ammonia is synthesized through a procedure known as the Haber Process, which involves both chemical and thermal energy in order to combine different gases to produce ammonia [3]. The first necessary ingredient is hydrogen, a product of water and natural gas, which is first subjected to a heat of 400॰C followed by an even higher temperature of 770॰C for purification [3]. After receiving a supply of nitrogen, the gas must cool to a low enough temperature where water condenses and may be removed, leaving just the pure ammonia gas [3]. Finally, kinetic energy comes into play, as the gas is subjected to high pressures and compressed into a liquid state, resulting in the product that is eventually used in making PAN fibers [3]. Although using natural gas as a power source is the most energy efficient means of synthesis, ammonia production (as a whole) still accounts for “about 17% of the energy consumed in [the U.S. industrial] sector, using up to “5.6 EJ [5.6 × 1018 J] of fossil fuels” [4]. To put that into perspective, 1 EJ alone is enough to power the average American home for about 25,806,185 years [5].
The second source material of PAN fibers is propylene, a substance obtained from a method called “cracking”, using reactant matter such as naphtha and gas oil [6]. In this case, cracking refers to breaking down hydrocarbon molecules into smaller, manageable, “more useful” ones, as compared to the conventional idea of cracking an object by simply fracturing it [7]. This method is commonly used as a means of extracting oil/petroleum-based substances, often inside a machine known as a tubular reactor [7]. These reactors operate using thermal energy, as a pipe superheats the reactants inside to temperatures reaching 1150 K in order to initiate the beginning chemical reactions [8]. Once those reactions start, chemical energy carries the rest of the process forward, as the thermal energy released during the reactions is contained and therefore reused, resulting in a sort of sustained loop [7]. Although heating the reactants to such high temperatures can be quite energy-intensive, over the last decade, the efficiency of cracking has drastically improved. With the development of technology like tubular reactors, which have become better at retaining and recycling heat released from chemical reactions, the amount of external energy sources needed, such as natural gas or electricity, has been reduced [7]. While I was able to find a significant number of reports backing the idea of more efficient cracking processes, I was unable to find specifics on the energy consumption rates or power outputs of the tubular reactors.
The final component used in crafting PAN is oxygen. Companies prefer to refine natural air samples and synthesize oxygen for industrial use through a process known as cryogenic air separation, which has steps similar to those taken when creating ammonia. After obtaining an air supply, manufacturers cool it to roughly -185॰C and compress it, removing any water vapor that may be present, leaving a gaseous mixture of nitrogen, oxygen, and argon [9]. With significantly different densities, nitrogen and oxygen are easily separable after small amounts of heat are applied to them [9]. Argon on the other hand, is much more difficult to fully remove from oxygen, meaning that small traces of argon may still be present in some of the purest oxygen samples [9].
Finally, the ammonia, propylene, and oxygen are all combined in a fluidized bed reactor, which utilizes both chemical and thermal energy [2]. Inside these machines, the initial substances are paired with a catalytic solvent, and the resulting chemical reactions produce heat, which then “controls the reaction rate” and polymerization of the product [10]. In order to prevent any outstanding flaws or defects in the polymer solution, the temperature must be kept at a constant 600 K, meaning a significant amount of energy is wasted as heat must be released from the reactor at a fast rate due to the reaction’s exothermic nature [2].
After polymerization, the material is subject to the spinning process, where it first submerges in a liquid bath. Known as “coagulation,” this step involves the transfer of thermal energy, as the newly formed PAN cools in the solution (made primarily of water) with a temperature of roughly 19॰C, which is maintained through the use of electrical power [11]. After coagulation, the PAN fibers are heated for thermal stabilization. As the name implies, this step uses thermal energy in the form of furnaces, which heat the fibers at temperatures ranging from 200°C to 260°C for approximately 90 minutes [12]. These industrial-grade furnaces are most often powered using natural gas; however, some companies prefer to use petroleum and other fossil fuels as well. [13]. After thermal treatment, the PAN fibers are finally complete, ready to arrive at ZOLTEK carbon fiber manufacturing plants.
Refined PAN fibers may be processed at any of the four ZOLTEK factories, with two located in Missouri, and the other two in Mexico and Hungary [14]. At these factories, the precursors undergo four different procedures, where they will eventually turn into carbon fibers. The first of these is called stabilization, which is ultimately just another instance of heating the fibers. With temperatures reaching 300°C, the thermal energy applied “causes the fibers to pick up oxygen molecules from the air,” which in turn actually transforms the atomic bonding of the precursors, resulting in a stronger, more stable “ladder formation” [15]. After initial heating for about two hours in a furnace, the fibers pass through a series of heated chambers and rollers. As will be a common trend with a majority of ZOLTEK production techniques, the details surrounding specific equipment or energy used are relatively vague, with many ZOLTEK reports simply stating that the company uses “a variety” of materials, reactants, and appliances, without clearly identifying them [15].
After stabilization, the precursors are heated once again during the carbonization phase. Here, a significant amount of thermal energy is required, for the fibers must be heated up to an extreme 3000 °C [15]. That being said, the precursors spend significantly less time in this process as compared to stabilization, only sitting in the furnaces for a few minutes [16]. The purpose of this brief but intense blast of heat is to burn away all non-carbon atoms in the precursor. The “remaining carbon atoms form tightly bonded..crystals,” resulting in the product’s immense trademark strength [15]. Not specific to ZOLTEK factories, but rather all carbon fiber manufacturers, carbonization uses .26 TBtu annually, or approximately 274,300,000 MJ [17]. Also calculated in this energy consumption total is the oxidation process, a simple yet significant step in producing carbon fiber. (That being said, a majority of the 274,300,000 MJ used is still from carbonization as oxidation does not require very much energy.) As indicated by its name, oxidation involves the joining of oxygen atoms to the recently carbonized fibers using kinetic energy in the form of air blasts, transforming some the bonds of the material as well as physically “etching” and “roughening” the surface [15]. The newly-formed texture allows coating and resin to better attach to the fibers.
The remaining step is called sizing, which involves coating the fibers as well as spinning them into yarn. ZOLTEK specifically uses epoxy, polyester, nylon, and urethane for coating [15]. Both thermal and kinetic energy are involved, as the materials must first be heated to their melting points to be suitable for coating, and the fibers must be mechanically drawn through them afterwards. Compared to the prior steps, sizing uses significantly less energy when heating because material melting points generally tend to be quite low. For instance, polyester, the most heat resistant material out of the four, has a melting point of only 295 °C [18]. In total, the entire sizing process uses only 31,650,000 MJ per year, which is admittedly a high consumption rate, yet is still much lower relative to processes such as stabilization or carbonization. With sizing complete and the fibers wound into yarn, the carbon fibers are finally ready for sale and distribution.
A wide array of companies use carbon fiber for their products which means the energy cost of distribution and transportation varies greatly, so I focussed my research on one of the most prominent uses of ZOLTEK carbon fiber: car body panels, specifically for Jeep and Cadillac models. With manufacturing plants in Arlington, Texas and Lansing, Michigan, GM most likely receives their supply of carbon fiber through semi-trucks, which require the use of fossil fuels in the form of gasoline [19]. On average, semi-trucks get approximately 7.2 MPG [20]. Considering the average distance between ZOLTEK factories in Missouri and GM manufacturing plants in Texas and Michigan is about 621 miles, just one shipment of carbon fiber requires over 85 gallons of gasoline, not accounting for any fluctuations in fuel economy as a result of upward slopes [21]. Once they reach their destination, the fibers are woven into cloth where they may be molded into solid sheets, in a process similar to sizing, where a coating or resin is used to help the fibers hold their shape [16]. Unfortunately for my research, GM keeps their specific fiber processing methods confidential, so I was unable to find specific energy data for cloth weaving or resin molding. Once they are in use, carbon fiber weaves themselves do not require energy, as they are simply used as building components of a larger product.
Until recently, carbon fiber repurposing was quite primitive by modern technological standards. The only way to separate the fibers from the resin mold was by exposing carbon fiber sheets to extremely high temperatures until the resin burned away.. This method not only results in a significant amount of lost heat and energy, but also renders the fibers structurally weaker than they once were, meaning they cannot be used for high intensity aerospace or automotive applications [22]. However, recent scientific developments have yielded a new means of recycling carbon fiber using chemical energy. With zinc chloride and liquid ethanol, researchers have been able to break the bonds between the carbon fiber and resin, which in turn allows the two components to be separated in near-perfect condition, preserving them both for reuse [23]. The process still uses thermal energy to help the acids enter the carbon fiber, but in much smaller amounts than simply burning off the resin does.
While there was a decent amount of transparency pertaining to ZOLTEK’s production procedures, the same cannot be said for specific appliances and mechanisms used in these processes. There were many instances in which I had to make assumptions using available data on commercial energy use or some generalizations obtained from the carbon fiber industry as a whole. Still, it is evident that although carbon fiber is not in the realm of harmful unsustainability, the product is far from being considered “green.” Such a strong, lightweight material comes at a notable cost, for synthesizing precursors as well as processing them into carbon fiber are highly energy-intensive. That being said, as the demand for the product continues to grow, so does the search for innovation and with it improvements in sustainability and energy efficiency, as seen through the newly developed recycling process.
References
[1] “What Is Carbon Fiber?” ZOLTEK, Toray Corporation, 23 Oct. 2017, zoltek.com/carbon-fiber/what-is-carbon-fiber/.
[2] Lazonby, John. “Poly(Propenonitrile) (Polyacrylonitrile).” The Essential Chemical Industry Online, University of York, 7 Oct. 2013, essentialchemicalindustry.org.
[3] Kirk, C. M., and J. C. Copplestone. “Ammonia and Urea Production.” Chemicals - Ammonia and Urea, nzic.org.nz/ChemProcesses/production/1A.pdf.
[4] “Ammonia.” Industrial Efficiency Technology & Measures, Institute for Industrial Productivity, 5 Sept. 2014, ietd.iipnetwork.org.
[5] “How Much Electricity Does an American Home Use?” FAQ, U.S. Energy Information Administration, 7 Nov. 2017, www.eia.gov.
[6] Lazonby, John. “Propene (Propylene).” The Essential Chemical Industry Online, University of York, 26 Jan. 2017, www.essentialchemicalindustry.org.
[7] Lichtarowicz, Marek. “Cracking and Related Refinery.” The Essential Chemical Industry Online, University of York, 7 Sept. 2014, www.essentialchemicalindustry.org.
[8] Lichtarowicz, Marek. “Chemical Reactors.” The Essential Chemical Industry Online, University of York, 18 Mar. 2013, www.essentialchemicalindustry.org.
[9] “Overview of Cryogenic Air Separation and Liquefier Systems.” Universal Industrial Gases, Inc, www.uigi.com.
[10] Park, Soo-Jin, and Gun-Young Heo. “Chapter 2 Precursors and Manufacturing of Carbon Fiber.” Carbon Fiber, 2015, p. 36., doi:10.1007/springerreference_208039.
[11] Morris, E., et al. “Properties of PAN Fibers Solution Spun into a Chilled Coagulation Bath at High Solvent Compositions.” Fibers, vol. 3, no. 4, 2015, p. 562., doi:10.3390/fib3040560.
[12] Ribeiro, Robson Fleming, et al. “Thermal Stabilization Study of Polyacrylonitrile Fiber Obtained by Extrusion.” PolÃMeros, vol. 25, no. 6, 2015, p. 526., doi:10.1590/0104-1428.1938.
[13] “Use of Natural Gas.” Energy Explained, Your Guide To Understanding Energy, Energy Information Administration, 26 Oct. 2017, www.eia.gov.
[14] “ZOLTEK Locations.” ZOLTEK, Toray Corporation, 24 Oct. 2017, zoltek.com.
[15] “How Is Carbon Fiber Made?” ZOLTEK, Toray Corporation, 23 Oct. 2017, zoltek.com.
[16] McConnell, Vicki. “The Making of Carbon Fiber.” CompositesWorld, CompositesWorld, 18 Dec. 2008, www.compositesworld.com.
[17] Bandwidth Study on Energy Use and Potential Energy Saving Opportunities in the Manufacturing of Lightweight Materials: Carbon Fiber Reinforced Polymer Composites. U.S. Department of Energy, 2015, www.energy.gov.
[18] “Polyester Fabrics.” Stern and Stern: Weaving Specialists, 2017, www.sternandstern.com.
[19] “General Motors Manufacturing Plants.” GM Authority, gmauthority.com/.
[20] Berg, Phil. “10 Things You Didn't Know About Semi Trucks.” Popular Mechanics, Popular Mechanics, 14 Nov. 2017, www.popularmechanics.com.
[21] “Google Maps.” Google, www.google.com/maps.
[22] Harris, Mark. “Carbon Fibre: the Wonder Material with a Dirty Secret.” The Guardian, Guardian News and Media, 22 Mar. 2017, www.theguardian.com.
[23] “New Way to Recycle Carbon Fiber Composites.” Materials Today, 9 May 2017, www.materialstoday.com.
Nick Cunningham
Design 40a
Winter 2018
March 11, 2018
Waste and Emissions: Carbon Fiber
Carbon fiber is one of the most innovative and advanced materials to be created. Made of an extremely strong composite weave, it has opened many doors for new applications in a variety of fields. It was first only used for aerospace engineering due to the high manufacturing costs and original difficulty to create, but is now used in everything from rockets to cars to home products. It’s a very (now) straightforward process to create it and doesn’t use many materials overall, with no massive physical harm to the earth’s surface such as digging or blasting. However, the waste and emissions created by the process that it is manufactured with are often overlooked, with pollution and used carbon fiber dumps being only a couple of examples of the problems of the creation of this composite. There have been upgrades to the recycling process to make it more cost effective and environmentally friendly that have begun to take advantage of leftover materials and reuse them for new things. This essay will venture into the often overlooked casualties of such a groundbreaking material: the environment, and see just what it costs to create carbon fiber.
Carbon fiber is made of thousands of long, extremely thin strands of carbon atoms arranged in a crystal pattern that are heated at around 200-300 degrees C to chemically change the structure of the fibers and then burned in non-oxygen gas mixture at temperatures from around 1000-3000 degrees C to remove most of the non carbon atoms, called carbonizing. They are then slightly oxidized to bond better and after they are coated with some form of protective material and spun onto bobbins to await being weaved into a sheet or whatever shape is desired or even used alone as yarn [1][2]. After, they are formed into that shape and coated in resin to maintain the shape and make it even stronger, forming a sheet of carbon fiber. The entire process is done on an assembly line powered by electricity as well as gas-powered engines for the furnaces that heat the carbon to give it such strong properties, creating large amounts of emissions and other toxic chemicals from the bonding process the fibers undergo.
Waste
Producing carbon fiber is a very specific process that, in the beginning, was quite inefficient; up to 30% of produced carbon fiber ending up as waste during the process and is basically just tossed out or partially recycled due to high costs [2]. Most of the total waste, however, comes after the product containing it has stopped being useful. Oddly shaped or special/custom carbon fiber products such as bike frames are not easily repurposed due to their unique shape, compounding the issue of waste because it is already difficult to recycle small pieces of this material. Carbon fiber dumps and landfills have become more and more prevalent as it has made its way into the mainstream, due to the difficulty of finding clear and efficient ways of recycling carbon fibers from old products. It isn’t biodegradable or photodegradable either, so when carbon fiber ends up in a landfill, it will be there for a very long time unfortunately. As it is made up of layers of composite materials to make a light and durable material, it’s extremely hard to recycle and reuse for it for other purposes on top of being non-biodegradable makes this composite difficult to justify environmentally.
One of the most common products to end up in landfills and dumps is the carbon bike frame. I’m specifically pointing this one item out because this seems to be the most widely and carelessly discarded items of carbon fiber in circulation. There are landfills made specifically for carbon bike frames and wheels that have thousands just piling up and some will have been sitting for years not being recycled or even considered for it because of the high energy and monetary costs associated with recycling carbon products[5]. This is a problem as well because bike frames are not common household items that can be easily just shined up and put back out to the market as a used item like a spoon or a chair; these bike frames are difficult to repurpose because of their odd shape and specialized use and it keeps many of them banished to the depths of landfills and dumps.
Emissions
The production of carbon fiber is done exclusively by machines, leading to an issue of emissions. Multiple toxic chemicals such as hydrogen cyanide (HCN), ammonia (NH3), and volatile organic compounds (VOCs) have the possibility to be emitted by the oxidation and carbonization furnaces and are immediately dangerous to humans within the vicinity even in small quantities. There are also other pollutants emitted by manufacturers are carbon monoxide (CO) and nitrous oxide (NOX) that are not only dangerous to humans, but also contribute to global warming [6]. The machines to make carbon fiber require enormous amounts of energy, specifically electricity and natural gas, and put out huge amounts of carbon dioxide-anywhere from 24-31 kg of CO2 per kg of carbon fiber [7]. To put that in perspective, in 2016 over 48,000 metric tons of carbon fiber was produced in just the U.S. and Mexico alone and production is continuing to increase each year [8]. This will only get worse as the years go on, with many executives expecting to see an increase in carbon fiber demand by up to another 100,000 metric tons by 2025, and the demand for it will outweigh the production capabilities of today's factories far more than the current demand, which is just above the world’s annual carbon fiber consumption [9]. In short, producing an extremely innovative material such as this comes at huge cost to the environment as well as the people near the factories and facilities due to the emissions put out by them. In terms of shipping carbon fiber products to either distributors or consumers, I was unable to find any numbers on the transport of the material. It is safe to say it is relatively high as they are most likely shipped on petrol and diesel truck/planes/boats which produce pretty large amounts of CO2 and other emissions.
Solutions
Although there have been a fair amount of issues related to the production of carbon fiber in terms of waste and emissions, there is good news. Companies and governments are starting to step up and ensure that the production process is safe and more energy efficient to help both people and the environment. Starting with waste, many production companies have starting investing more in solutions to recycle used carbon products or the waste products generated in original production with relatively new processes. The most common and cheapest ways of recycling carbon fiber are through pyrolysis, which is burning off the resin coating of carbon fiber in an oxygen-less environment and leaving just the fibers, the most effective way to preserve the strands and keep them long. Longer strands are far stronger than short strands so keeping them as long as possible ios always the goal and pyrolysis is the most effective way to keep the best strands to be reused in products that require strength such as cars or planes. The other most common way to reclaim carbon fiber strands is shredding. Shredding is quite straightforward, using industrial shredders to chop up products and basically ripping the resin coating off of the fibers. However, this does shorten the fibers, making them less resilient to impacts and are only able to be used in products that do not require any kind of impact ratings. Recycling of carbon fiber is still a relatively new process that is still being perfected to use less energy and maintain longer strands and will most likely take a while to optimize completely. [10]
The emissions produced by carbon fiber manufacturers are by far the biggest drawback of the industry, with huge amounts of carbon dioxide and other less than desirable gases being released into the air. Luckily, governments and companies themselves have begun trying to minimize the emissions put out by their carbon furnaces and ovens in an attempt to help both the environment and the people within the industry. Companies such as Anguil are working to develop air pollution control systems that can massively reduce operating costs for carbon fiber furnaces. By using leftover heat generated by the oxidation process, it will continue the combustion but also supplement the heat in order to maintain it at a high temperature while lowering the electricity costs overall by removing the need for a secondary heating device as well as achieving a 99% destruction rate efficiency rating for the oxidation process. This company has also developed a new oxidizer that destroys the necessary nitrogen compounds for the oxidation process with little to no NOX generation as a byproduct and therefore almost completely removing the risk of a toxic chemical being produced regularly and in large quantities. They have also developed an added option for that oxidizer called SNCR or Selective Non-Catalytic Reduction to further reduce the environmental impact of the furnace by destroying VOCs and HAPs [11] [12]. This oxidizer has also been improved with an induced draft system to draw Hydrogen Cyanide into the oxidizer and destroying it [12].
Carbon fiber is an extremely valuable material in today's society; it’s strong, light, and extremely durable as well as being able to take nearly any shape that is required of it. It’s been used in products from spaceships to shoe horns and everything in between because of that space age appeal and the expensive science used to make it. It does create a bit of waste and the emissions from its creation is a huge negative for it due to the amount of CO2 and other toxic chemicals that are naturally made from superheating some elements. Luckily for humans and the environment, improvements are being made everyday to mitigate or remove these problems and help to bring such a powerful and necessary to today's consumer market.
Sources Cited:
[1]“Carbon Fibers.” Wikipedia, Wikimedia Foundation, 10 Mar. 2018, en.wikipedia.org/wiki/Carbon_fibers.
[2]“How Is Carbon Fiber Made?” ZOLTEK, 23 Oct. 2017, zoltek.com/carbon-fiber/how-is-carbon-fiber-made/.
[3]Barnes, Frazer. “Recycled Carbon Fiber: Its Time Has Come.” CompositesWorld, CompositesWorld, 29 June 2016, www.compositesworld.com/columns/recycled-carbon-fiber-its-time-has-come-.
[4]Suciu, Peter, et al. “The Not so Green Bike: Carbon Fiber's Carbon Footprint.” BikeRadar, 17 Dec. 2011, www.bikeradar.com/us/gear/article/the-not-so-green-bike-carbon-fibers-carbon-footprint-31898/.
[5]“Photos of Carbon Fibre Bike Dump Reveal Worrying Waste Levels.” Sticky Bottle, 14 Dec. 2015, www.stickybottle.com/latest-news/photos-of-carbon-fibre-bike-dump-reveal-worrying-waste-levels/.
[6]“New Clean Air Techniques for Carbon Fiber Processes.” Anguil Environmental Systems, Inc., www.anguil.com/news/new-clean-air-techniques-for-carbon-fiber-processes/.
[7]Cook, Jefferey J. “Carbon Fiber Manufacturing Facility Siting and Policy Considerations: International Comparison.” 2017, www.nrel.gov/docs/fy17osti/66875.pdf.
[8] “Carbon Fiber Production Capacity Top Countries 2017 | Statistic.”Statista, www.statista.com/statistics/380549/leading-countries-by-carbon-fiber-production-capacity/.
[9]Sloan, Jeff. “Carbon Fiber 2016 Report.” CompositesWorld, CompositesWorld, 30 Dec. 2016, www.compositesworld.com/articles/carbon-fiber-2016-report.
[10]RecycleNation. “Is Carbon Fiber Better for the Environment than Steel?” RecycleNation, 21 Oct. 2015, recyclenation.com/2015/10/is-carbon-fiber-better-for-environment-than-steel/.
[11“New Clean Air Techniques for Carbon Fiber Processes.” Anguil Environmental Systems, Inc., www.anguil.com/news/new-clean-air-techniques-for-carbon-fiber-processes/.
[12]Systems, Sponsored by Anguil Environmental. “Controlling Emissions during Carbon Fiber Manufacturing – Oven and Furnace Emissions Control.” AZoM.com, 1 Aug. 2017, www.azom.com/article.aspx?ArticleID=10910.