Design Life-Cycle
assess.design.(don't)consume
DES 40A
Ziqra I. Raza
Christina Cogdell
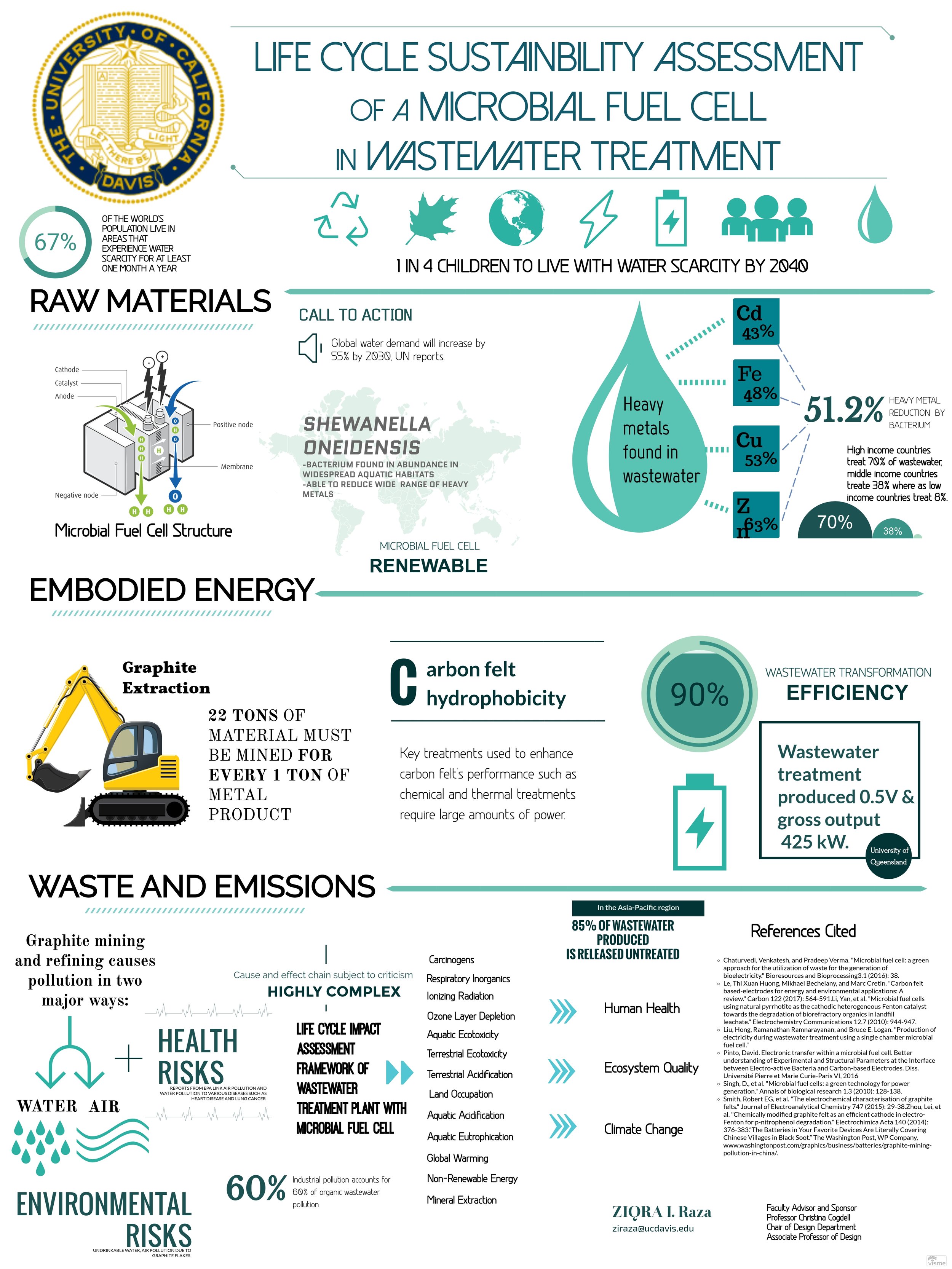
Raw Materials – Microbial Fuel Cell
World research nowadays centers around the need to discover and develop new clean and green technologies that reduce our dependence on non-renewable forms of energy. As the demand for energy increases, the demand for finite resources also increases. However their supply is dwindling. On top of that, the retrieval process as well as the processing of these high energy density sources have huge environmental consequences. This is why we turn to renewable resources, energy we know will never run out. And so this is where fuel cells come in, an example of a green sustainable technology.
Fuel cells are electrochemical devices that continuously generate energy by converting chemical energy to electrical energy. It consists of anodic and cathodic tanks that are supplied with combustible fuel (these differ from batteries which need to be recharged, as opposed to a fuel cell that functions with an ever present fuel source[1]). Key components that make up a microbial fuel cell are the electrode materials, a fuel source (in this life cycle analysis is wastewater) and the biocatalyst.
In order for an electrode to be effective, the material that will be chosen to act as the electrode must balance the following properties: high conductivity, specific surface area, porosity, low sensibility to corrosion and bio-corrosion (for the case of a microbial fuel cell), and the electrode cannot foul the bacteria that will be used to inoculate the anode[2]. Other industry determining factors also include cheap, availability and ability to enlarge the scaling.
The material carbon felt is often used an electrode material due to its good electronic conduction, high surface area, biocompatibility, non-toxicity, chemical stability, low sensibility to corrosion and porosity[3]. These characteristics allow for the electrode to be an effective redox reaction site, have excellent electrolytic efficiency and mechanical stability. What's even more is that the material boasts these properties at a relatively low cost. Carbon felt based electrodes have been widely used for redox flow batteries (RFBs) and wastewater treatment by electrochemical advanced oxidation processes (EAOPs)[4].
Another major raw material associated with the microbial fuel cell is of course the bacterium itself. Shewanella oneidensis was first discovered from the sediments of Lake Oneida in New York in 1988. This bacterium was geographically named Shewanella oneidensis MR-1 for Manganese reducing 1. This bacterium is predominantly found in deep sea anaerobic habitats. It can also be found in soil and sedentary habitats.
The salt bridge is also a key component in the functioning of a MFC. The salt bridge allows for the flow of charged ion between the two separated cells of the fuel cell. It maintains electrical neutrality within the circuit, preventing the fuel cell from running its reaction to equilibrium in a short amount of time. A common type of salt bridge is the agar salt bridge.
Embodied Energy
There is an increasing global demand in energy, and in order to resolve this growing problem a source of sustainable and environmentally friendly energy is needed. This is where microbial fuel cells come in as a potent solution as a sustainable and green bioenergy conversion technology that literally lives off of waste. Not only is fossil fuel depletion of dire concern in terms of not being able to meet future energy demands, but there is also global interest in developing environmentally friendly green technology. Bioenergy is one of the solutions that is currently being widely researched as an answer to this dilemma. Microbial fuel cells are electrochemical devices that take advantage of a microorganism’s metabolic process, which directly converts organic matter into electricity. Microbial fuel cells are cost effective (as they operate under ambient environment conditions with regards to temperature and pressure), require no external energy input, and possess ease of mobility (unlike hydrogen fuel cells).
Microbial fuel cells employ microorganisms as the biocatalysts instead of metal catalysts, which are widely applied in conventional fuel cells. Microbial fuel cells are capable of generating electricity as long as nutrition is provided. It converts chemical energy of organic and inorganic fuels into electrical energy. However, to industrialist’s dismay, miniature microbial fuel cells have faster power generation recovery than macroscale microbial fuel cells[5]. On the flip side, since power generation density is affected by the surface-to-volume ratio, miniature microbial fuel cells can facilitate higher power density[6].
When it comes to the embodied energy of the microbial fuel cell, the extraction of one of the major raw materials, graphite, contributes largely to the life cycle of the MFC. Graphite is the natural form of carbon[7]. It is characterized by its hexagonal crystalline structure and is extracted using open pit and underground mining methods. Graphite extraction is based off of the degree of weathering the graphite ore’s rock has undergone, as well as the proximity of the graphite ore to the earth’s surface[8]. The most common one is the open pit method (surface quarrying), as the types of graphite that are involved with this method of extraction are flake graphite and macro-crystalline graphite. The types of graphite have distinct characteristics as a result of their occurrences in different geolocation regions. As a note, global demand for flake graphite and macro-crystalline graphite is the highest.
Open pit mining, has to do with extracting rock or minerals from a burrow, or an open pit of some sort. This method is employed when the graphite ore is close to the earth’s surface and the surface material that covers the graphite deposit is thin enough to penetrate into. Minerals found deep underground, where the solid is overstrained (minerals will appear to be vein-like in the hard rock), underground mining methods are carried out (requires a significantly higher amount of energy which is commercially not favored).
Quarrying is the form of surface mining used to obtain graphite. The rocks are broken by either drilling or using dynamite explosives. These processes then cut the rocks open. Compressed air or water is then used to split the rocks. Bore hole mining is another way to obtain graphite, where a hole is drilled into the earth to reach the ore. A tube is then used to push water through and pump it back up to make a slurry. This slurry, made up of the water and mineral is then pumped to a storage tank for further processing. Usually drilling and blasting methods are used on hard rock ore to loosen the large sized graphite flakes attached to the graphite rock. These flakes are then crushed to the ground before they go airborne as they are subject to floatation[9]. This part of the extracted graphite is then brought to the surface with locomotives. In developing countries however, such as China, the graphite flakes are handpicked, shoveled and pulled in a cart that is then moved to a plan for further processing. Thus dependent on the geographic location of the mining, the embodied energy differs for the microbial fuel cell. Unfortunately numbers of the energy difference could not be obtained due to the lack of transparency of the mining processes.
Most walls of the open pit are dug at angles, like terraces. So the angle is less vertical to avert and lessen damage and hazards from rock falls. The angle varies with the level of weathering the rocks have undergone, the kind of rock and its structural weakness such as faults, shear strains, joints and foliations. So the walls are stepped, where the inclined part is called the batter and flat part is called the bench or perm. The making of these steps on the hill require heavy machinery as well (except in lesser developed countries where these processes are more labor intensive[10]). The steps help the rocks from falling down. There is a haul road usually located at the side of the pit in the form of a ramp which trucks drive on to carry away the materials, which again contributes to the embodied energy of the microbial fuel cell.
The Kearney graphite mine in Ontario follows a similar process[11]. The graphite rock ore will be used to extract graphite from using the open pit mine method. The graphite obtained is then trucked to the processing mills. The ore is crushed in a crushing circuit which comprises of a jaw crusher, and is then transported to the hydraulically driven semi-autogenous grinding mill via conveyors. The graphite is screened, grinded and crushed, after which the material passes through a floatation circuit where it is cleaned and polished. The exhaust heat that from the generators is used to dry the concentrate of the graphite rock. Their website also says that the radiated heat provides warmth during winter, not specifically for who or what though. The graphite that will be used for the market is then packed into a one tone bag and trucked away for delivery, while the waste rock (non-graphite bearing rock) and is then deposited at the waste rock area which is then shifted to a tailings storage facility.
The embodied energy in the microbial fuel cell in terms of its materials, and how its components allow for the cell to harness such energies can be explored via the carbon felt electrodes. The porosity of the carbon felt, its permeability, is dependent on the hydrophobic nature of the felt itself. This value is based off of the Darcy law (velocity of a fluid via a volume unit of a porous medium)[12]. This value tells us about how easy it is for a fluid to pass through a porous material. The better the porosity of a material means that it will have a higher permeability coefficient as the fluid will flow more easily. Carbon felt however, does have a downside to it. The felt material has high levels of hydrophobicity.
Figure 1: Hydrophobicity of carbon felt
This reduces the permeability of the material as water droplets rest on the surface. Chemical treatments are usually thus carried out to modify the material at the surface to eliminate this barrier. While there are expensive and energy intensive methods used for this step such as pulsed laser deposition, there are also cheaper and more environmentally friendly methods such as simply using phosphoric acid[13].
Thermal treatment is another simple way to modify the felt to allow for improvements in its electrochemical properties and hydrophilicity. The enhanced electrochemical properties of the modified electrodes lead to better electrical conductivity because of the increased number of active sites on the electrode, as well as the decrease in its hydrophilic properties[14]. Heat treatment also improves the surface area of the felts, by roughly ten times, thereby increasing the efficiency of the cell as well. However heating it at high temperatures like 450˚C adds to the embodied energy of the fuel cell life cycle negatively. But this is a method used to enhance the efficiency of the fuel cell even more than what is already has.
Thus we can see the excellent electrical conductivity, chemical stability, lightweight, low cost, are all properties the carbon felt possess. Unfortunately it is the carbon felt’s hydroponic nature that makes its application most difficult in aqueous electrolyte mediums[15]. A way to overcome this drawback is through chemical and heat treatments as discussed above. Although this adds to the cost of manufacturing for the fuel cell, the enhanced wettability of the electrodes makes it easier for the electrolyte ions to travel to the active sites of the electrode. This improves the conductivity at the electroactive surface. Microbial fuel cells also want to have extremely good bio-compatibility, in order to grow the bacteria on the felt electrode. Researchers argue that the low cost of the bacterium offsets the high costs of the chemical and heat treatments necessary for the efficient functioning of the microbial fuel cell[16].
The manufacturing method used to create carbon fibers from graphite flakes is known as needle-punching[17]. Carbon felt, a 3D organization of randomly aligned fibers, compared to carbon rods in a two chamber microbial fuel cell (where the anode was inoculated with bacterium) revealed a three time increase in the current. Thus the felts were more effective than the carbon rods. This current increase was correlated with the higher specific area and the bacteria quantity at the surface[18].
This process is an important step which decides the internal structure, textile structure or thickness homogeneity of the felts. The needle’s barbs hook onto the fibers and then insert them vertically. Rearranging the fibers and the layers leads to reinforcement of the fibers through enhanced compact layers and better integrated fibers. Thermal treatment at around 1200-1600˚C then follows the needle punching process which is a downside to the making of carbon felt.
Figure 2: Needle punching process https://www.sciencedirect.com/science/article/pii/S0008622317306577#bib17>
Current wastewater treatment option are energy intensive and environmentally unfriendly, thus there have been vast efforts to try and discover new approaches for wastewater treatment. One of the methods involves bio-electrochemical systems, particularly the microbial fuel cell. Whether or not the microbial fuel cell provides a significant environmental benefit with regards to the environmental impact it would have for wastewater treatment vs the making of the fuel cell itself is to be explored.
Bio electrochemical systems are known for combining the advantages or treating wastewater and producing electrical current at the same time. However, in order to achieve similar energy recovery performance as conventional fuel cells, Rabaey and Verstraete[19] have said that microbial fuel cells have to achieve a power density of 1000W.m-3 during wastewater treatment, at a loading rate of 10kg. According to their paper, these densities have not yet been reached. But using synthetic substrates, highly conductive media and very small reactors reported power densities of the desired order. Currently research is being carried out to try and demonstrate similar results for more realistically scaled microbial fuel cells.
Below are process flow diagrams of wastewater treatment options. Most of the options bear strong similarities to each other.
Figure 3: Wastewater treatment processes https://pubs.acs.org/doi/pdf/10.1021/es100125h
Firstly, the raw wastewater is pumped through screens and flows into the balance tank. Here the water undergoes preacidification for 12h which is done through doses of citric acid and/or caustic soda (for pH correction purposes). In treatment option 2, the microbial fuel cell, the wastewater then underwent treatment in fuel cell itself and produced electricity at a current density of 1000 A. m-3 and a voltage of 0.5V [20].This electricity that was generated by the fuel cell was then transformed to grid voltage and was used to power equipment on site. The energy here was 423 kW gross, which included 90% transformation efficiency from the 0.5V. This ended up replacing the standard grid electricity as the basis of a microbial fuel cell pilot-scale experiment conducted at the University of Queensland set assumed the operational energy requirements of the microbial fuel cell were 0.1 kW . m-3 .
The final post treatment stage involves the dissolved air filtration step before disposal to sewer and downstream treatment in municipal wastewater treatment plants. Polymer and ferric chloride are added to the dissolved air filtration step to help with the solid capture. These waste solids were then dewatered using a centrifuge and then disposed by road transport to landfills.
The pretreatment phase of the wastewater had little associations with the end point impacts as approximately 90% (insert footnote) of the total life cycle impacts arose from the electricity consumption and the operational phase (transportation/ disposal of bio solids). Thus improvements in energy efficiency and process optimization for this stage alone will not have a significant impact in reducing the environmental impacts involved with the waste water treatment. The positive environmental impact that came out of this was the replacement of the electricity grid, which was large enough to outweigh the negative impacts thereby presenting an overall net environmental benefit (only in this European context). This paper highlighted that environmental benefits can be achieved by displacing fossil fuel based electricity generation (72% of the UK electricity mix is from coal, oil and natural gas[21]) with electricity generated from microbial fuel cell technology in wastewater treatment processes. The microbial fuel cell generated 0.40 kg CO2-e per kWh, compared to 0.56 kg CO2-e per kWh for UK grid electricity[22]. So the major drawback is the resource and emissions intensive materials required for the construction of the microbial fuel cell itself (involves carbon felt, membrane materials, PVC, stainless steel, etc.). However, assessing the waste and emissions involved with the making of the microbial fuel cell will provide researchers with the opportunity to select and develop appropriate and environmentally benign materials for the construction of the microbial fuel cell.
Waste and Emissions
In the digital era we live in today, with new emerging technologies popping up daily, one can easily get caught up with the fast pace of tech development and lose sight of some basic things such as ethics. With regard to the microbial fuel cell, although it boasts the title of being clean and green, just because the functioning of the fuel cell is environmentally friendly does not necessarily mean the processes underwent to make the fuel cell itself were safe and sustainable. The waste and emissions that come hand in hand with the making of the microbial fuel cell, particularly the carbon felt electrodes will be explored below. Additionally we will also look at the green side of the microbial fuel cell, and how it can be used for wastewater treatment, thanks to the anode being inoculated with the bacterium Shewanella Oneidensis MR-1.
Unfortunately there is a plethora of negative outcomes that come about due to the mining of graphite (material used for carbon purposes such as making carbon felt electrodes). In this day and age, graphite has become an indispensable resource, where it is an ingredient used in almost any electronic device (including microbial fuel cells). The irony here is underscored when companies promote their products on the landscapes of green energy and clean technology, with their futuristic plans to lead us into a pollution free world. So at the moment there are two sides to the coin, where one is a green future but the other is old fashioned industrial pollution. This factor of the carbon electrode plays a huge role the microbial fuel cell in terms of waste and emissions.
Graphite, carbon, is a key ingredient in batteries that power phones, electric cars, etc. A large number of graphite mining is done in China as although graphite is found all over the world, Heilongjiang province, which lies on the Russian border, is the largest single source. The Washington Post conducted case studies in five towns in two provinces of China ( one of which was Heilongjiang) where they saw a similar trend in the villages living near graphite companies: damaged crops, homes covered in soot, polluted drinking water and dust filled air. Near these factories villagers fear for their health, and protests that have been underway have been futile[23]. China is notoriously known for dust polluting its air and water, and now we know a large part of it is due to the graphite mines.
Tesla, one of the world's best known electric car makers has declined to identify a graphite source for their batteries. Although they have said that they use Panasonic batteries for their cars and have also said that Panasonic does not buy their graphite from graphite supply giant company, Chinese company BTR[24]. This Chinese company serves roughly 75% of the market demand for natural graphite materials for batteries, according to Chen Bifeng remarks, marketing director for BTR in an interview with The Post. So they have large footing in the supply chain of the graphite market[25]. However when it comes to making the battery there are a large number of intermediate companies that take part in the making of the battery such as the battery anodes. An example company is Shanshan, a Chinese anode making company that has refused the Post any remarks.
China dominates the graphite industry largely because of its pricing. Graphite is not exclusively only found in China, however the low costs Chinese companies offer it at gives them the competitive advantage in the market, thus discouraging other companies to open mines elsewhere. The price of raw graphite that can be used for refinement is about $550. President of U.S based Asbury Carbons (began importing graphite from China in the 1970s), Stephen A. Riddle, said that ‘price, purity and quantity’ were the reasons why China was such a giant in the graphite market. Chinese companies, with their combination of low labor costs and ingenuity of mining graphite attracted a large fraction of the buyers, said Riddle. In fact, in the 1970’s China produced roughly a tenth of the world’s supply of graphite. And by 2015, according to the U.S geological Survey, China was producing about two-thirds of the world’s supply of graphite. In the early 1980s Riddle visited one of the mines and saw the workers working with picks and shovels to obtain the raw material from the dirt. Whereas in more developed countries tractor and other heavy equipment were employed. ‘They were obviously a very low-cost operation’, according to Riddle.
The environmental consequences associated with their production are massive. Lyu Shengwan, a laborer aged 55 interviewed by the Post said ‘they mine anywhere on the mountain that they want to…the plants release their discharge into the water.’ He said the water is undrinkable and that now the village retrieves water from a source more than a mile away. As the demand for more powerful laptops, tablet, phones and other electronic devices rises (inevitable in this digital era), the demand for graphite rises in parallel.
Without proper rules and regulations, the mining and refining of graphite can cause pollution by air and water. Graphite powder is something that is easily airborne leading to fine particle pollution. This causes breathing problems, worsening lung diseases or just reducing lung function overall. The U.S Environmental Protection Agency has reported links to heart attacks in people with heart disease and breathing in polluted air. Graphite mining also leads to water pollution because the chemicals used for purification, like hydrofluoric acid, leak into the water. This highly toxic substance not only affects the biodiversity but this water is no longer drinkable for the local people either.
Riddle said that this method of purification is cheaper, by 15% than the more environment friendly way to purify graphite, which is through heat treatment, or ‘baking’.
Now the general consensus would correlate economic success with higher living standards of the people of the country, and economic disparity with low living standards. In China’s case however, we see the opposite, where decades of impressive economic growth has lead to the Chinese government showing increasing concern for its nations environmental and health woes. A project run by the University of Washington known as the Global Burden of disease found that a million or more Chinese die prematurely every year because of outdoor pollution. China Water Risk, a non-profit group that tallies figures from China’s Ministry of Environmental Protection found that 60% of the groundwater in China was classified as ‘bad’ or ‘very bad’. Additionally more than a quarter of China’s key rivers was classified as ‘unfit for human contact’ (China Water Risk depicted this from the figures the government provided). Now as China is known to be global producer of many raw materials, industry is woven deep into the land. Hence it is difficult to pinpoint the exact amount graphite industries contribute to the water pollution. Graphite industries in China have been fined, however there are also factories that haven’t been. Two of the five factories The Post journalist reported on have no government records with the Institute of Public and Environmental Affairs (Beijing non-profit). Residents, and some industry representatives say that, although the government wants to protect the environment, there is also a matter of the government wanting to protect jobs. Some argue that it’s better than being unemployed.
It’s also important to note that it isn’t just China that is affected. According to research published in 2014 in the Proceedings of the National Academy of Sciences, over a quarter of the emissions of two key pollutants in China (sulfur dioxide and nitrogen oxides) arose because of the increase in the production of goods for exports. This is because of the rising demand for exports worldwide. Hence outsourcing production to China doesn’t rid a country of the environmental problems it would have to face if the production were to take place on their land. Plus with the ever so rising demand for high speed electronics, a solution is needed fast.
With regards to the microbial fuel cell, we have focused on the beginning part of the life cycle, which has to do with the raw materials. Now if we look at the fuel cell in action, there is a positive side (which whether or not outweighs the waste and emissions from the raw material manufacturing is still being explored), and this has to do with the bacterium inoculated into the anode, Shewanella Oneidensis MR-1. This bacterium has the ability to reduce heavy metals as it means of ‘breathing’ and produce electricity at the same time. “Shewanella has naturally evolved to live in environments that contain no oxygen’ said Zach Rengert, a member of the research team, Bazan Research Group, ‘…it’s essentially the opposite of rust formation.’[26] This is what makes Shewanella Oneidensis MR-1 a great candidate for wastewater treatment, as while it removes heavy metal contaminants, at the same time it produces energy in an oxygen free environment. Research has shown that MFC’s can reduce up to 80% of the organic material present in sewage wastewater. So essentially, you’re using waste to clean up waste. Microbial fuel cells harness the power of bacteria produce electrical energy. The bacteria breaks down wastes and sewage (as its mode of survival) and generates an electric current in the due process. MFC’s are self-sustaining as well since the bacteria replicates on its own and produces power indefinitely as long as it has a food source to live off of. This is an example of green energy, where we do not rely on fossil fuels or other non-renewable sources of energy.
A pilot case study was conducted over 10 years of operation, based at a full-scale operating wastewater treatment plant[27]. From this functional unit we can see that the first order environmental impacts include the direct atmospheric emissions and effluent discharges. There are also second-order impacts: the emissions and resources required for upstream electricity generation and chemical manufacture. It is largely the construction and operating phases that contribute largely to the impacts[28].
Figure 4: System Boundary image of the water treatment process source - Foley, J.; De Haas, D.; Hartley, K.; Lant, P. Comprehensive Life Cycle Inventories of Alternative Wastewater Treatment Systems. Water Res. 2010, 44 (4), 1654–1666
Figure 5: source Foley, J.; De Haas, D.; Hartley, K.; Lant, P. Comprehensive Life Cycle Inventories of Alternative Wastewater Treatment Systems. Water Res. 2010, 44 (4), 1654–1666
Here we can see the cause and effect chain of environmental processes and mechanisms. End point impacts in attempting to quantify outcomes in terms of tangible damage (loss of resources, loss of species, affected life years) are highly complex thus the modeling is highly subject to criticism. Additionally, in this paper, the background inventory data used was entirely European based, thus the conclusions drawn are with respect to this context.
Works Cited
Chaturvedi, Venkatesh, and Pradeep Verma. "Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity." Bioresources and Bioprocessing3.1 (2016): 38.
Chouler, Jon, et al. "Towards effective small scale microbial fuel cells for energy generation from urine." Electrochimica Acta 192 (2016): 89-98.
Dannys, E., et al. "Wastewater treatment with microbial fuel cells: a design and feasibility study for scale-up in microbreweries." J Bioprocess Biotech 6.267 (2016): 2.
Le, Thi Xuan Huong, Mikhael Bechelany, and Marc Cretin. "Carbon felt based-electrodes for energy and environmental applications: A review." Carbon 122 (2017): 564-591.
Li, Yan, et al. "Microbial fuel cells using natural pyrrhotite as the cathodic heterogeneous Fenton catalyst towards the degradation of biorefractory organics in landfill leachate." Electrochemistry Communications 12.7 (2010): 944-947.
Liu, Hong, Ramanathan Ramnarayanan, and Bruce E. Logan. "Production of electricity during wastewater treatment using a single chamber microbial fuel cell." Environmental science & technology 38.7 (2004): 2281-2285.
Logan, Bruce E., and John M. Regan. "Microbial fuel cells—challenges and applications." (2006): 5172-5180.
Nimje, Vanita Roshan, et al. "Comparative bioelectricity production from various wastewaters in microbial fuel cells using mixed cultures and a pure strain of Shewanella oneidensis." Bioresource technology 104 (2012): 315-323.
Pandey, B. K., V. Mishra, and S. Agrawal. "Production of bio-electricity during wastewater treatment using a single chamber microbial fuel cell." International Journal of Engineering, Science and Technology 3.4 (2011).
Pinto, David. Electronic transfer within a microbial fuel cell. Better understanding of Experimental and Structural Parameters at the Interface between Electro-active Bacteria and Carbon-based Electrodes. Diss. Université Pierre et Marie Curie-Paris VI, 2016.
Singh, D., et al. "Microbial fuel cells: a green technology for power generation." Annals of biological research 1.3 (2010): 128-138.
Smith, Robert EG, et al. "The electrochemical characterisation of graphite felts." Journal of Electroanalytical Chemistry 747 (2015): 29-38.
Zhou, Lei, et al. "Chemically modified graphite felt as an efficient cathode in electro-Fenton for p-nitrophenol degradation." Electrochimica Acta 140 (2014): 376-383.
“The Batteries in Your Favorite Devices Are Literally Covering Chinese Villages in Black Soot.” The Washington Post, WP Company, www.washingtonpost.com/graphics/business/batteries/graphite-mining-pollution-in-china/.
[1] https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Carbon+felt+based-electrodes+for+energy+and+environmental+applications%3A+A+review&btnG=#d=gs_cit&p=&u=%2Fscholar%3Fq%3Dinfo%3AxwoP8ZqD2U4J%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den>
[2] https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Carbon+felt+based-electrodes+for+energy+and+environmental+applications%3A+A+review&btnG=#d=gs_cit&p=&u=%2Fscholar%3Fq%3Dinfo%3AxwoP8ZqD2U4J%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den>
[3] https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Carbon+felt+based-electrodes+for+energy+and+environmental+applications%3A+A+review&btnG=#d=gs_cit&p=&u=%2Fscholar%3Fq%3Dinfo%3AxwoP8ZqD2U4J%3Ascholar.google.com%2F%26output%3Dcite%26scirp%3D0%26hl%3Den
[4] H. Liu. R. Ramnarayanan. B. E. Logan. “Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell.” Environmental Science & Technology, vol. 38, iss. 7, pp. 2281-2285, 2004
[5] H. Liu. R. Ramnarayanan. B. E. Logan. “Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell.” Environmental Science & Technology, vol. 38, iss. 7, pp. 2281-2285, 2004.
[6] http://illumin.usc.edu/134/microbial-fuel-cells-generating-power-from-waste/>
[7] http://www.greatmining.com/open-pit-mining.html
[8] http://www.greatmining.com/open-pit-mining.html
[9]http://www.greatmining.com/open-pit-mining.html
[10] https://www.mining-technology.com/projects/kearney-graphite-mine-ontario/
[11] https://www.mining-technology.com/projects/kearney-graphite-mine-ontario/
[12] https://fracfocus.org/groundwater-protection/fluid-flow-subsurface-darcys-law
[13] Chakrabarti, Mohammed Harun, et al. "Application of waste derived activated carbon felt electrodes in minimizing NaCl use for electrochemical disinfection of water." Int. J. Electrochem. Sci 6 (2011): 4470-4480.
[14] Gorby, Yuri A., et al. "Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms." Proceedings of the National Academy of Sciences 103.30 (2006): 11358-11363.
[15] Logan, Bruce E. "Exoelectrogenic bacteria that power microbial fuel cells." Nature Reviews Microbiology 7.5 (2009): 375.
[16] Nevin, Kelly P., et al. "Power output and columbic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbial fuel cells." Environmental microbiology 10.10 (2008): 2505-2514.
[17] Ringeisen, Bradley R., et al. "High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10." Environmental science & technology 40.8 (2006): 2629-2634.
[18] Saheb-Alam, Soroush, et al. "Effect of start-up strategies and electrode materials on carbon dioxide reduction on biocathodes." Applied and environmental microbiology 84.4 (2018): e02242-17.
[19] Rabaey, K.; Rodriguez, J.; Blackall, L. L.; Keller, J.; Gross, P.; Batstone, D.; Verstraete, W.; Nealson, K. H. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 2007, 1 (1), 9–18
[20] Rozendal, R. A.; Hamelers, H. V. M.; Rabaey, K.; Keller, J.; Buisman, C. J. N. Towards practical implementation of bioelectrochemical wastewater treatment.Trends Biotechnol. 2008, 26 (8), 450–459
[21] Hospido, A.; Teresa Moreira, M.; Martin, M.; Rigola, M.; Feijoo, G. Environmental Evaluation of Different Treatment Processes for Sludge from Urban Wastewater Treatments: Anaerobic Digestion versus Thermal Processes. Int. J. Life Cycle Assess. 2005, 10 (5), 336–345
[22] Foley, J.; De Haas, D.; Hartley, K.; Lant, P. Comprehensive Life Cycle Inventories of Alternative Wastewater Treatment Systems. Water Res. 2010, 44 (4), 1654–1666
[23] https://www.washingtonpost.com/graphics/business/batteries/graphite-mining-pollution-in-china/
[24] https://www.washingtonpost.com/graphics/business/batteries/graphite-mining-pollution-in-china/
[25] https://www.washingtonpost.com/graphics/business/batteries/graphite-mining-pollution-in-china/
[26] Logan, Bruce E., and John M. Regan. "Microbial fuel cells—challenges and applications." (2006): 5172-5180.
[27] Foley, J.; De Haas, D.; Hartley, K.; Lant, P. Comprehensive Life Cycle Inventories of Alternative Wastewater Treatment Systems. Water Res. 2010, 44 (4), 1654–1666
[28] Foley, J.; De Haas, D.; Hartley, K.; Lant, P. Comprehensive Life Cycle Inventories of Alternative Wastewater Treatment Systems. Water Res. 2010, 44 (4), 1654–1666